Antibody of TNF (Tumor Necrosis Factor) alpha and application thereof
A technology of antibodies and antigens, applied in the direction of antibodies, applications, anti-inflammatory agents, etc., can solve the problems of insufficient affinity of monoclonal antibodies, cannot be used in clinical practice, and high production costs, achieve important economic value and social significance, and improve inhibition TNFα ability, effect of enhancing affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Materials and methods:
[0030] Recombinant human tumor necrosis factor TNFα was purchased from PeprotechAsia (Remcombinant Human TNF-α, Cat: 300-01A).
[0031] The phage display kit was purchased from Amersham Bioscience (Recombinant phage antibody system, Cat: 27-9401-01).
[0032] See SEQ ID NO:151 for the heavy chain sequence of D2E7. The D2E7 light chain sequence is shown in SEQ ID NO:152.
[0033] Linker sequence between heavy and light chains (SEQ ID NO: 138):
[0034] 5'-GGTGGAGGCGGTTCAGGTGGAGGCGGTTCAGGTGGAGGCGGTTCA-3', encoded amino acid sequence (SEQ ID NO: 135): 5'-(Gly 4 Ser) 3 -3', a total of 15 amino acids.
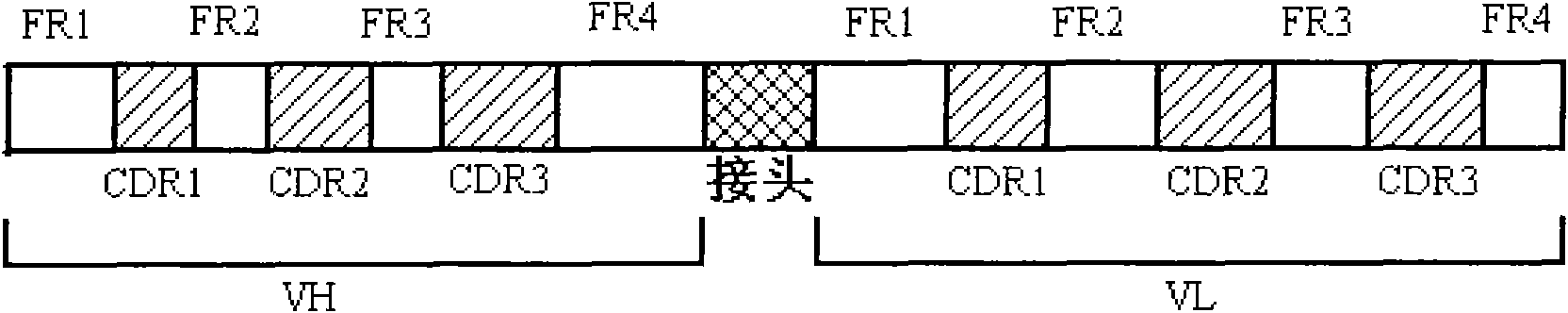
[0035] First, according to the heavy chain and light chain of D2E7 and the sequence of linker (linker), the complete nucleic acid sequence is artificially synthesized in the following order: (V H ) FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4-linker sequence-(V L )FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4 (D2E7scFv), see figure 1 .
[0036] Secondly, primers were synth...
Embodiment 2
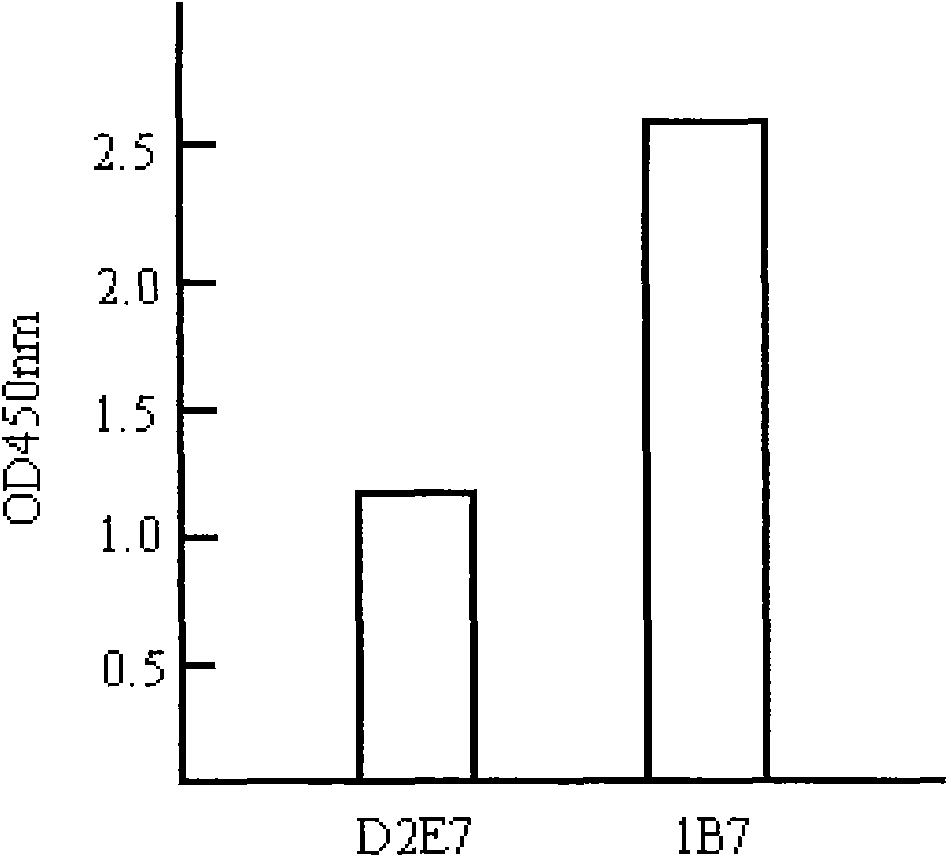
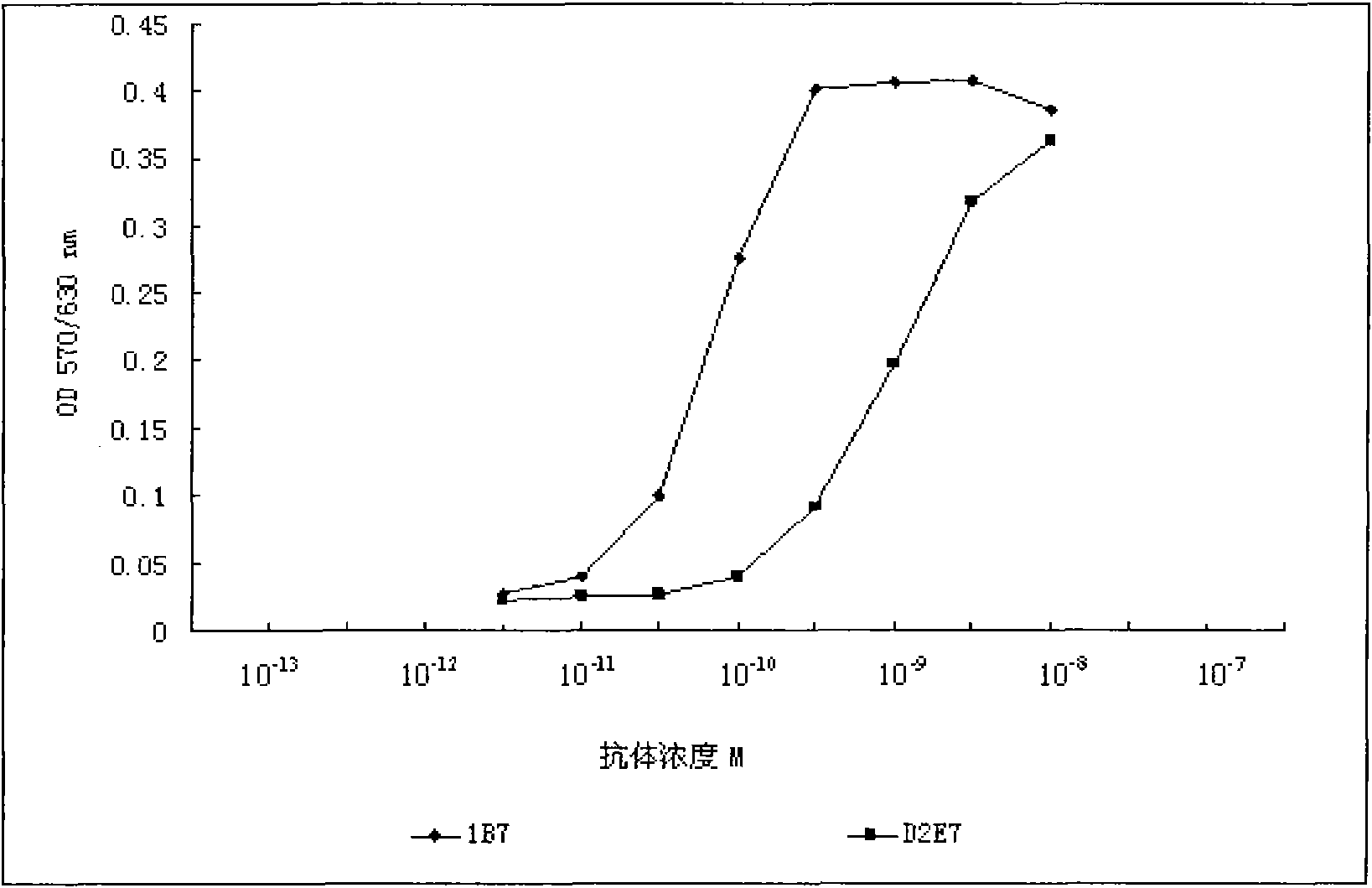
[0228] Taking 1B7 as an example, the effect of each mutant amino acid on its affinity was further studied in depth. Compared with the wild-type D2E7, clone 1B7 has mutated one amino acid in each CDR region of the heavy chain and the light chain, and mutated 6 amino acids in total. Each mutated amino acid is back-mutated, that is, the mutated amino acid is restored. It is a wild-type sequence, and the results of phage Elisa show that the amino acid reversion (His to Ile) mutation in the heavy chain CDR2 region does not affect the affinity of the antibody, but the other 5 amino acid reversion mutations will affect the affinity of the antibody. In other words, the remaining 5 amino acid mutations can all improve the affinity of D2E7.
[0229] The mutation method and the phage Elisa method are described above.
Embodiment 3
[0230] Example 3: Expression and purification of full-length antibodies
[0231] Materials and methods:
[0232] Chinese hamster ovary cells CHO were purchased from ATCC, recombinant protein A rProtein A was purchased from GE Healthcare Bio-Sciences AB (rProtein A Sepharose Fast Flow, Cat: 17-1279-03), protein G (Protein G) was purchased from GE Healthcare Bio-Sciences AB (rProtein A Sepharose 4 FastFlow, Cat: 17-0618-06).
[0233] Full-length antibodies, including the variable and constant regions of the heavy chain (human gamma1, gamma2, gamma3, gamma4 regions), and the variable and constant regions of the light chain (human kappa or lambda region), usually need to be produced in lactation Animal cell expression has therapeutic activity. Commonly used mammalian cells include CHO, NSO, COS, SP2, 293 and other cells. The heavy chain and light chain of an antibody can be expressed separately on different vectors, or expressed on the same vector. Vectors usually require scre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com