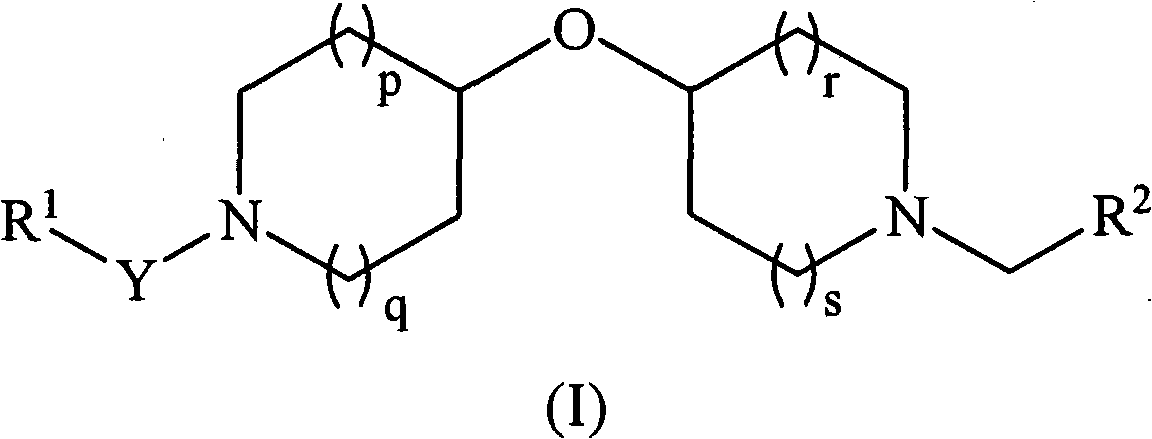
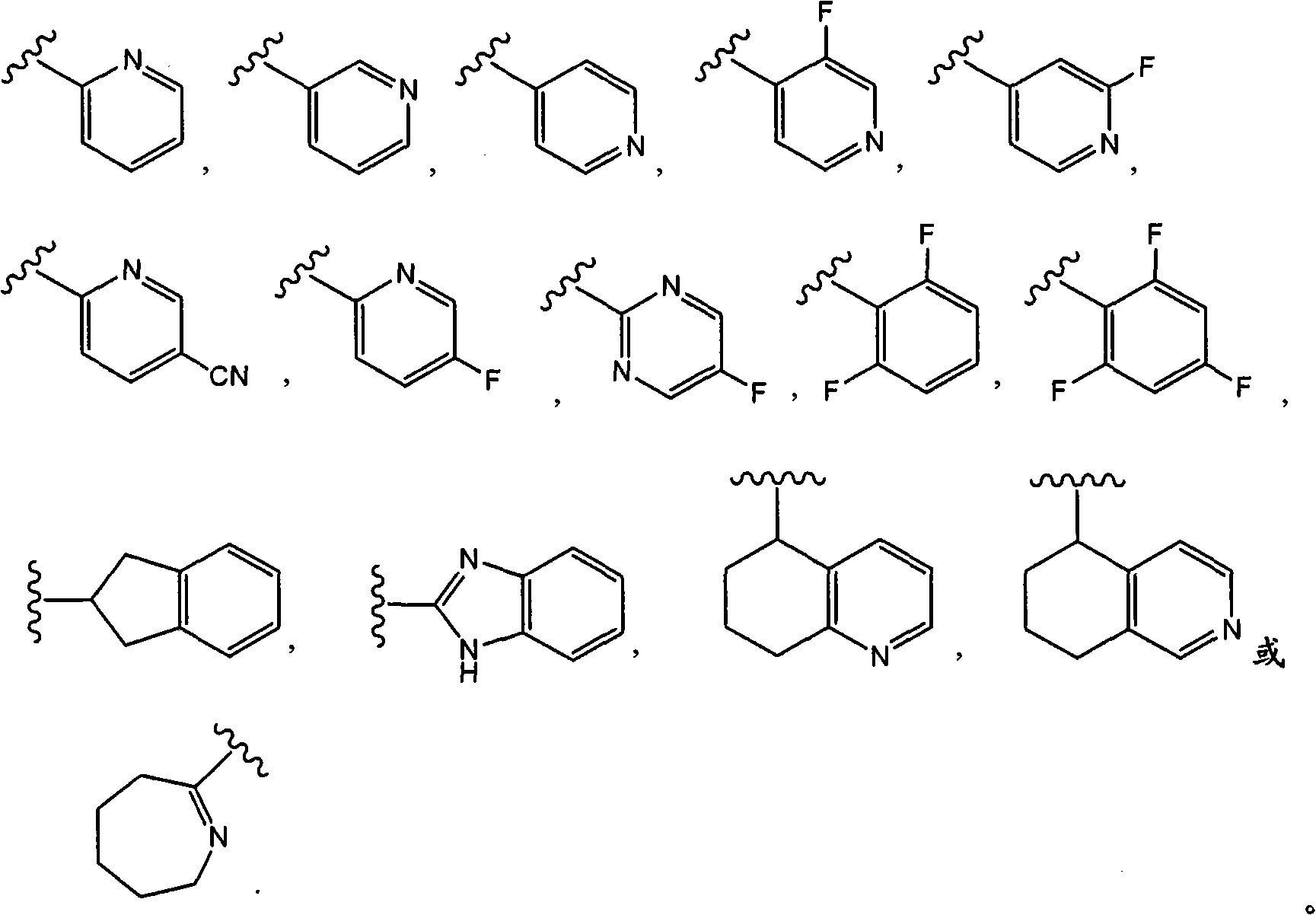
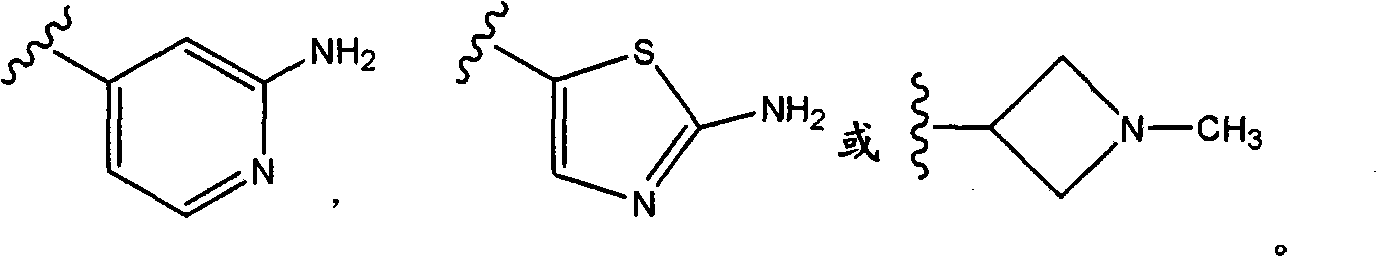
Oxypiperidine derivatives and methods of use thereof
A solvate and medicament technology, applied in the field of oxygen piperidine derivatives as histamine receptor antagonists, can solve problems such as nausea/diarrhea, lactic acidosis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[0386] Preparation of Compound 4
[0387]
[0388] Step A
[0389] To a stirred solution of 4-hydroxypyridine (2 g, 21.03 mmol) in 70 mL of anhydrous THF was added 4-hydroxypiperidine (5.29 g, 26.28 mmol) at room temperature. Triphenylphosphine (6.9 g, 26.31 mmol) was then added followed by diisopropylazodicarboxylate (5.2 mL, 26.41 mmol) dropwise. The reaction was heated to 55°C and allowed to stir at this temperature for approximately 15 hours. The reaction mixture was then cooled to room temperature and concentrated in vacuo. The resulting oily residue was treated with 1.0M aqueous HCl (30 mL) and washed with CH 2 Cl 2 (30 ml x 2) wash the acidic solution. Merged CH 2 Cl 2 Wash with 1.0M aqueous HCl (10 mL) and H 2 O (20 mL) was re-extracted and discarded. The combined aqueous fractions were basified to pH~12 using 1.0M aqueous NaOH, and the basic solution was washed with CH 2 Cl 2 (50ml x4) extraction. The combined organic extracts were washed with brine, ...
Embodiment 2
[0401] Preparation of intermediate compound 2B
[0402]
[0403] Step A
[0404] Using the procedure described in Example 1, Step C, bis-N-heterocycloalkyl ether 1B (196 mg, 0.689 mmol) was converted to compound 2A (155 mg, 58%).
[0405] Step B
[0406] Using the procedure described in Example 1, Step D, amide 2A (155 mg) was converted to compound 2B (96.4 mg, 86.5%).
Embodiment 3 and 4
[0408] Preparation of intermediate compounds 3A and 4A
[0409] Using the procedure described in Example 2, except using the acids specified in the table below, compound IB was converted to intermediate compounds 3A and 4A.
[0410]
[0411]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com