Trimeric IL-1Ra
A technology of trimerization and fusion protein, which can be used in DNA/RNA fragments, allergic diseases, metabolic diseases, etc., and can solve problems such as the inability to block the action of IL-1α
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
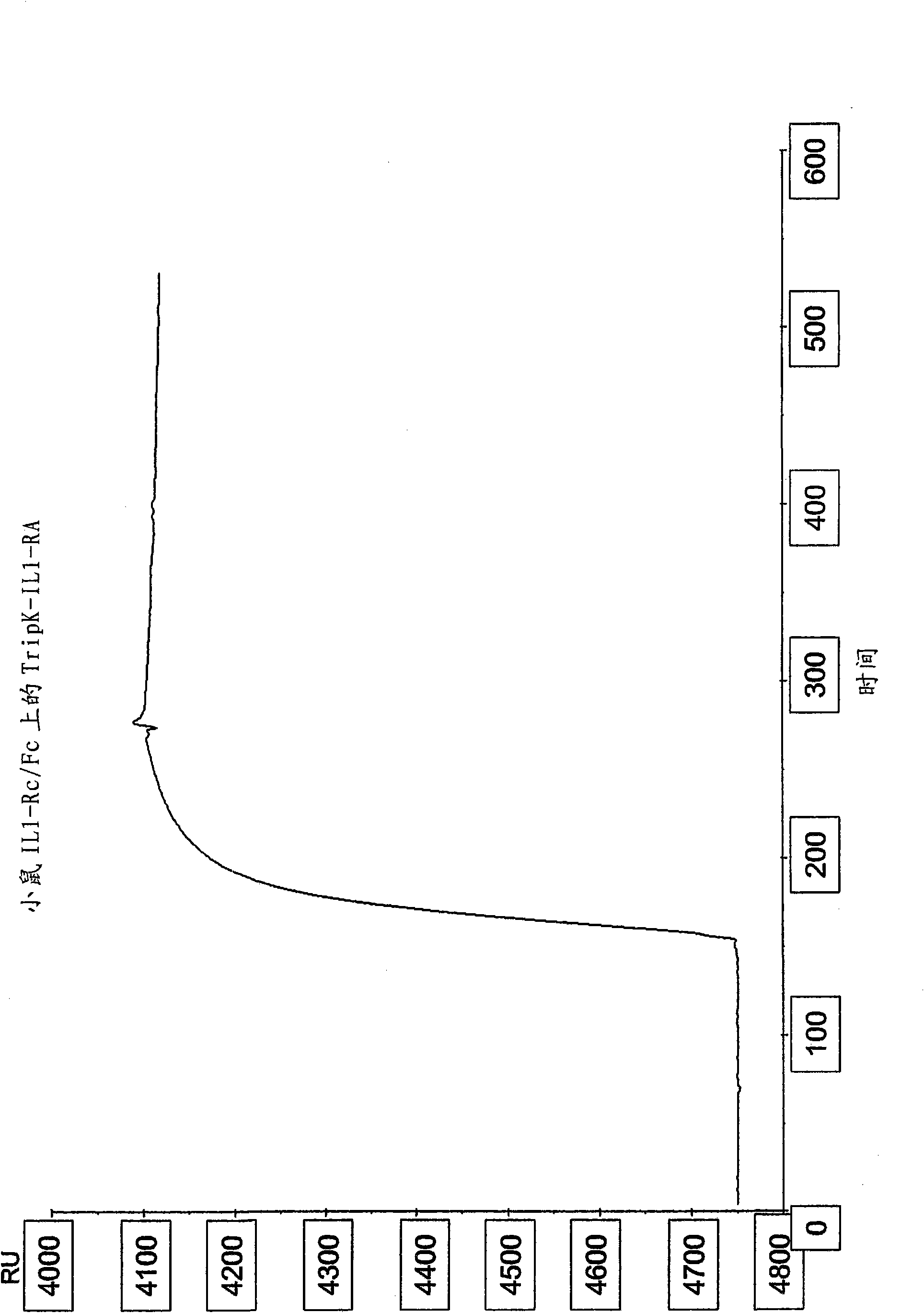
[0135] Example 1: Formation, production and purification of trimeric IL-IRa
[0136] It has been previously shown that IL-IRa can be produced as a recombinant protein in E. coli (Steinkasserer et al. 1992. FEBS 310:63-65). The protein is very stable and refolds efficiently. Isoforms of IL-IRa with additional amino acids at the N-terminus have also been described (Haskill et al. 1991, PNAS 88:3681-3685; Muzio et al. 1995, JEM 182, 623-628). These molecules bind IL-1R as well as the mature secreted form, suggesting that it may be possible to fuse an excess peptide to the N-terminus of the antagonist without affecting binding to the receptor. Structural analysis of crystals of IL-IRa that interacts with IL-IR also supports that N-terminal changes do not affect interaction with ILIR (Schreuder et al. 1997, Nature 386: 190-194). IL-IRa was cloned from human cDNA libraries derived from bone marrow and / or human placenta.
[0137] Trimeric IL-IRa was engineered to be C-terminally...
Embodiment 2
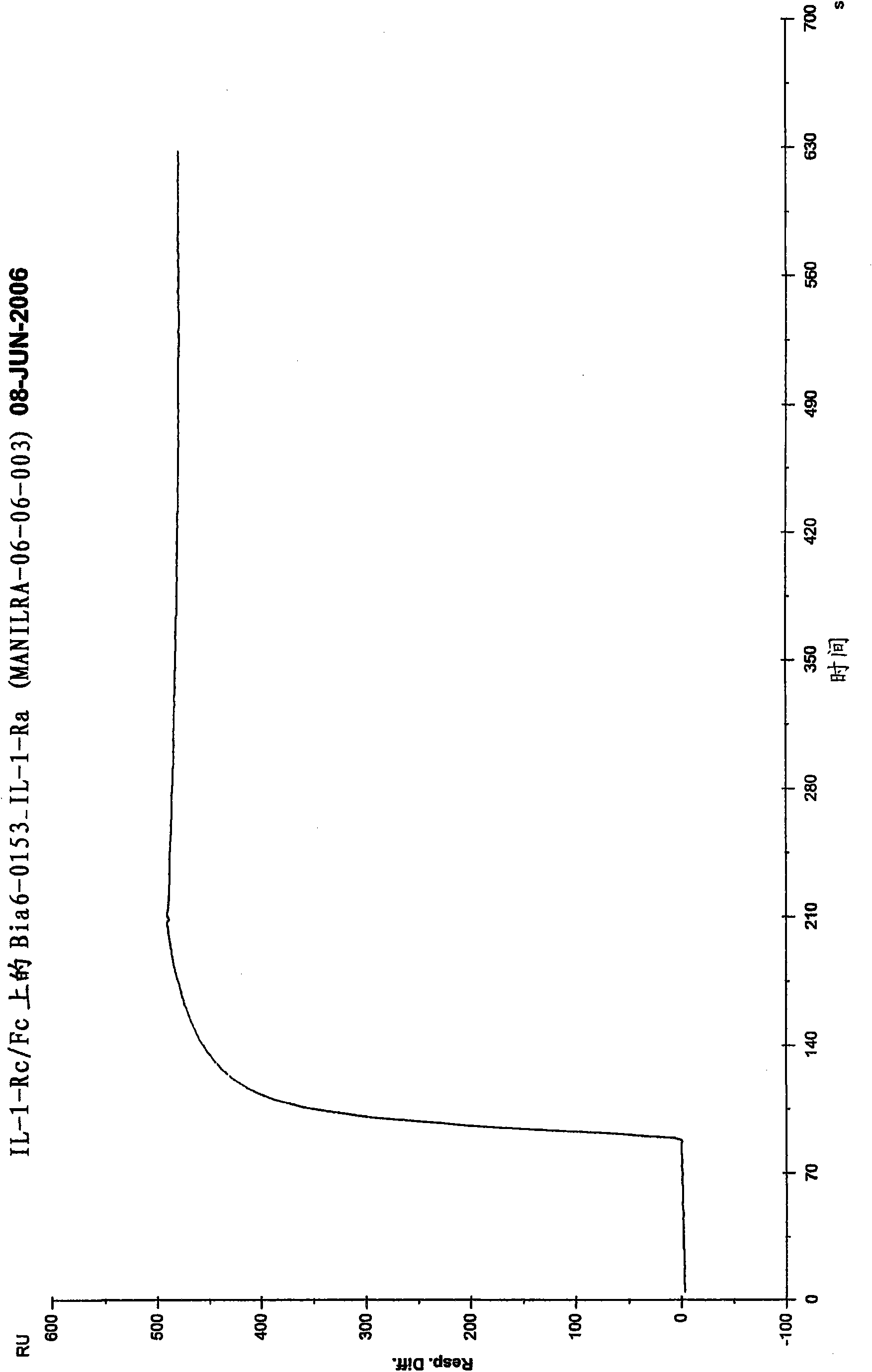
[0156] Example 2: Trimeric IL-1Ra compound inhibits IL-1 induction of IL-8 in U937 cells ability to guide
[0157] Further analysis of GG-TripV-IL-1ra (trip V-IL1Ra), GG-TripK-IL-1ra (tripK-IL1Ra), GG-TripT-IL-1ra (trip T-IL1Ra) and CII-H6-GrB-GG - Ability of TripT-IL-1ra (trip Q-IL1Ra) to inhibit IL-1 induction of IL-8 in U937 cells. The results are shown in Figure 4 middle.
[0158] Compounds were essentially equally potent in the blocking response and all exhibited (comparing w / w) works just as well. IL-8 production increased rather than continued to decrease at the highest protein concentration used (100 g / mL) due to the buffering effect in the experiment. According to several in vitro effect detection experiments and Biacore detection, according to the blocking and binding efficiency and yield, it is determined that TripT IL1Ra is the best compound.
Embodiment 3
[0159] Example 3: Polyethylene Glycol Trimeric IL-1Ra Compounds
[0160] Because the in vivo half-life is KINERET Key parameters in efficiency (KINERET The half-life in humans is only 4-6 hours, and thus can be used once a day), so the ability to pegylate TripT IL1Ra by N-terminal pegylation was tested. Trimeric IL1-Ra was PEGylated at the N-terminus. The trimeric IL1-Ra antagonist protein after the last step of the purification procedure described above was used as the starting point for pegylation. The buffer was exchanged with PBS buffer pH 6.0 for pegylation reaction. The protein concentration in the reactions was 0.5-3.5 mg / ml and a 5-10 molar excess of mPeg5K-acetaldehyde or mPeg20K-acetaldehyde (Nektar) supplemented with 20 mM sodium borocyanide was used. The reaction was carried out at 20°C for 16 hours. After the reaction, the mixture was applied to a Source 15S column (GE Healthcare) to purify the mono-PEGylated form. Such as Figure 5 As shown, the antagon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com