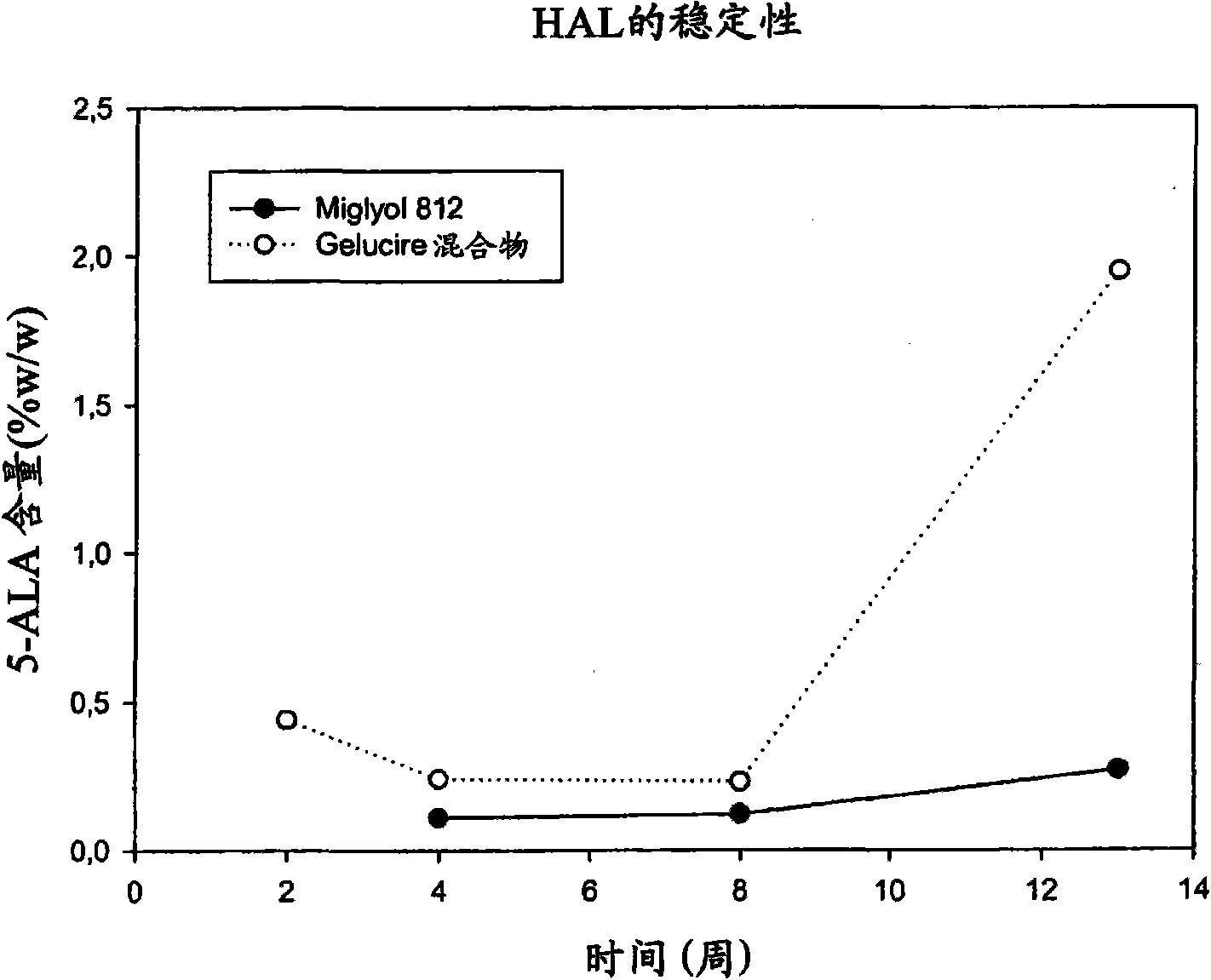
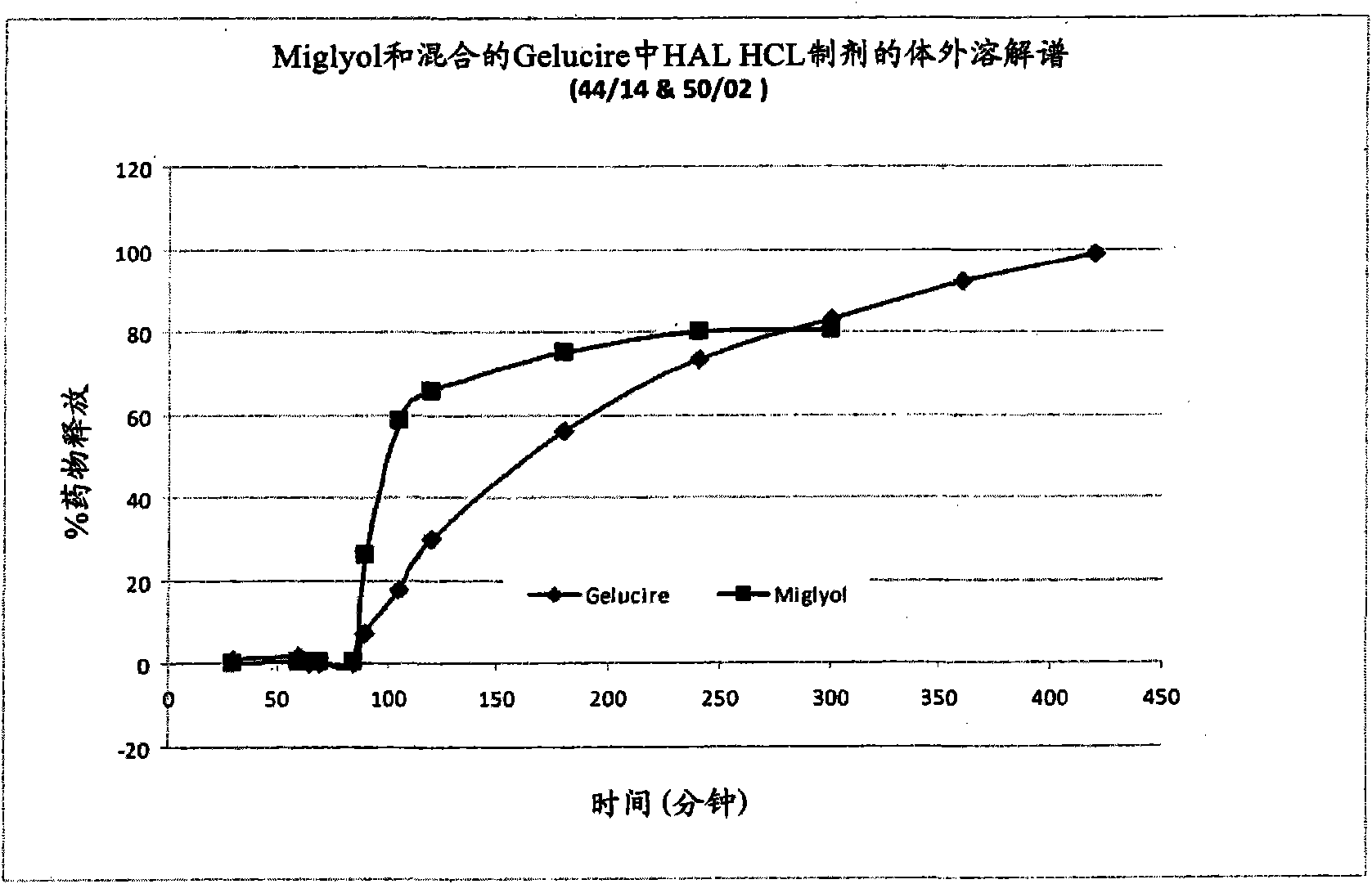
Use of 5-aminolevulinic acid and derivatives in a solid form for photodynamic treatment and diagnosis
A technology of aminolevulinic acid and photodynamic therapy, which is applied in the field of photodynamic therapy and diagnosis of diseases such as cancer, and can solve problems such as the reduction of the efficacy of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1 - the suppository containing 5-ALA hexyl ester
[0115] Each suppository (2g) contains:
[0116] 5-aminolevulinic acid hexyl ester hydrochloride (HAL HCl) 100mg, 10mg or 0.8mg, ethylenediaminetetraacetic acid disodium salt (EDTA) 40mg
[0117] Appropriate amount of Witepsol S 55 or S 58
[0118] Suppositories are prepared by suspending HAL HCl and ethylenediaminetetraacetic acid disodium salt in liquid Witepsol. The mixture is filled into molded and cooled suppositories.
Embodiment 2
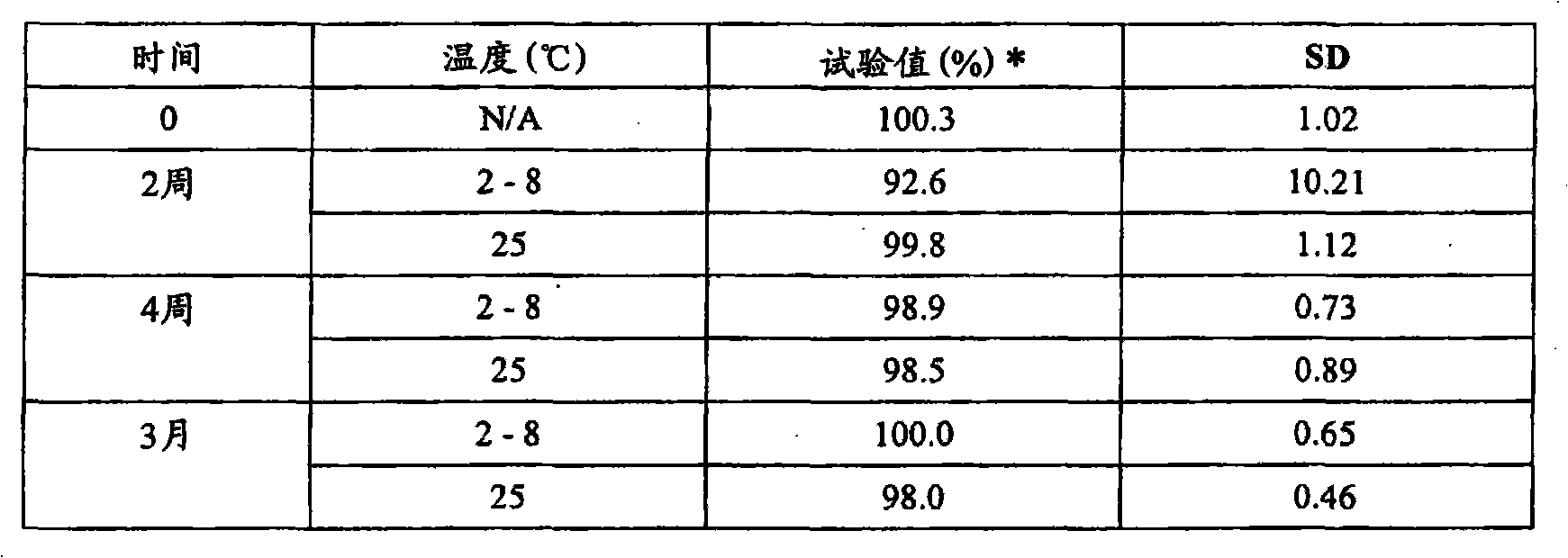
[0119] Example 2 - Stability of 5-ALA hexyl ester in suppositories, based on Witepsol S55
[0120] Suppositories containing hexyl 5-aminolevulinate hydrochloride (HAL HCl) were prepared as described in Example 1. The stability of HAL HCl in suppositories (based on Witepsol S55) was investigated by HPLC analysis. Stability was tested at room temperature (25°C) and freezer temperature (2-8°C). The results are shown in Table 1 below.
[0121] Table 1 Stability of 100 mg HAL HCl, based on Witepsol S 55
[0122]
[0123] * The test value is calculated as % of the theoretical concentration of HAL HCl in the preparation
[0124] The results in Table 1 show that suppositories containing HAL HCl (based on Witepsol S55) are stable for at least 3 months at room and refrigerator temperatures.
Embodiment 3
[0125] Example 3 - Stability of 5-ALA hexyl ester in suppositories, based on Witepsol S58
[0126] Suppositories containing hexyl 5-aminolevulinate hydrochloride (HAL HCl) were prepared as described in Example 1. The stability of HAL HCl in suppositories (based on Witepsol S58) was studied by HPLC analysis. Stability was tested at room temperature (25°C) and freezer temperature (2-8°C). The results are shown in Table 2 below.
[0127] Table 2. Stability of 100 mg HAL HCl, based on Witepsol S 58
[0128]
[0129] * The test value is calculated as % of the theoretical concentration of HAL HCl in the preparation
[0130] The results in Table 2 show that suppositories containing HAL HCl (based on Witepsol S58) are stable for at least 3 months at room and refrigerator temperatures.
[0131] Reference ratio
[0132] Four batches of aqueous cream containing 160 mg / g methyl 5-aminolevulinate (MAL) were left at room temperature (25° C.) for 3 months and their MAL content w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com