Method for detecting compound salvia miltiorrhiza tablet
A technology for compound Danshen tablet and detection method, which is applied to measurement devices, pharmaceutical formulations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
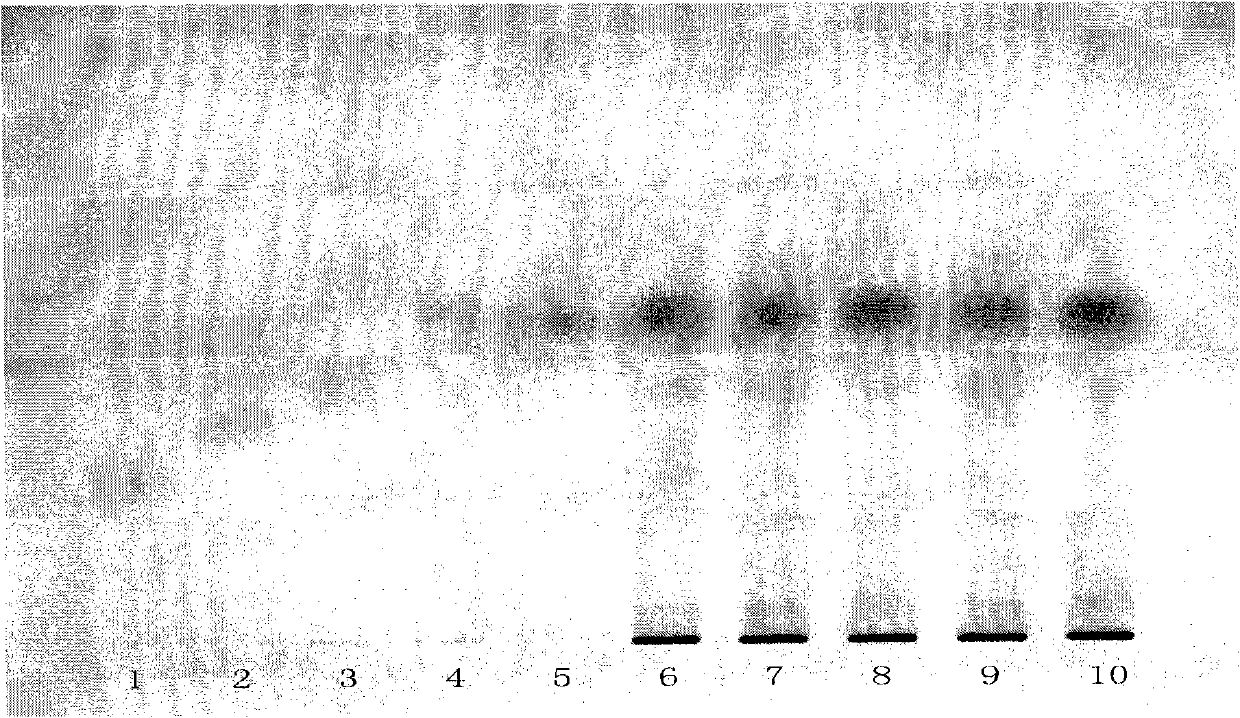
[0042] According to the specification requirements of a Compound Danshen Tablet in the Pharmacopoeia of the People's Republic of China in 2010, 2 batches of specifications (1) (5 tablets in each batch, respectively numbered 6 and 7), 2 batches of specifications (3) (5 tablets in each batch) , respectively numbered 8, 9), 1 batch of specifications (2) (2 pieces, numbered 10).
[0043] Grind the above 2 batches of specifications (1) 5 tablets per batch, 2 batches of specifications (2) 5 tablets per batch and 1 batch of specifications (3) 2 tablets with sugar coating removed, add 10ml of ether each, ultrasonicate for 5 minutes, filter After that, discard the filter residue, evaporate the filtrate to dryness, add 2ml of ethyl acetate to the residue to dissolve, and use it as the test solution respectively (the corresponding numbers are 6, 7, 8, 9, 10). Take another dihydrotanshinone Ⅰ reference substance (number 1), cryptotanshinone reference substance (number 2), tanshinone Ⅰ ref...
Embodiment 2
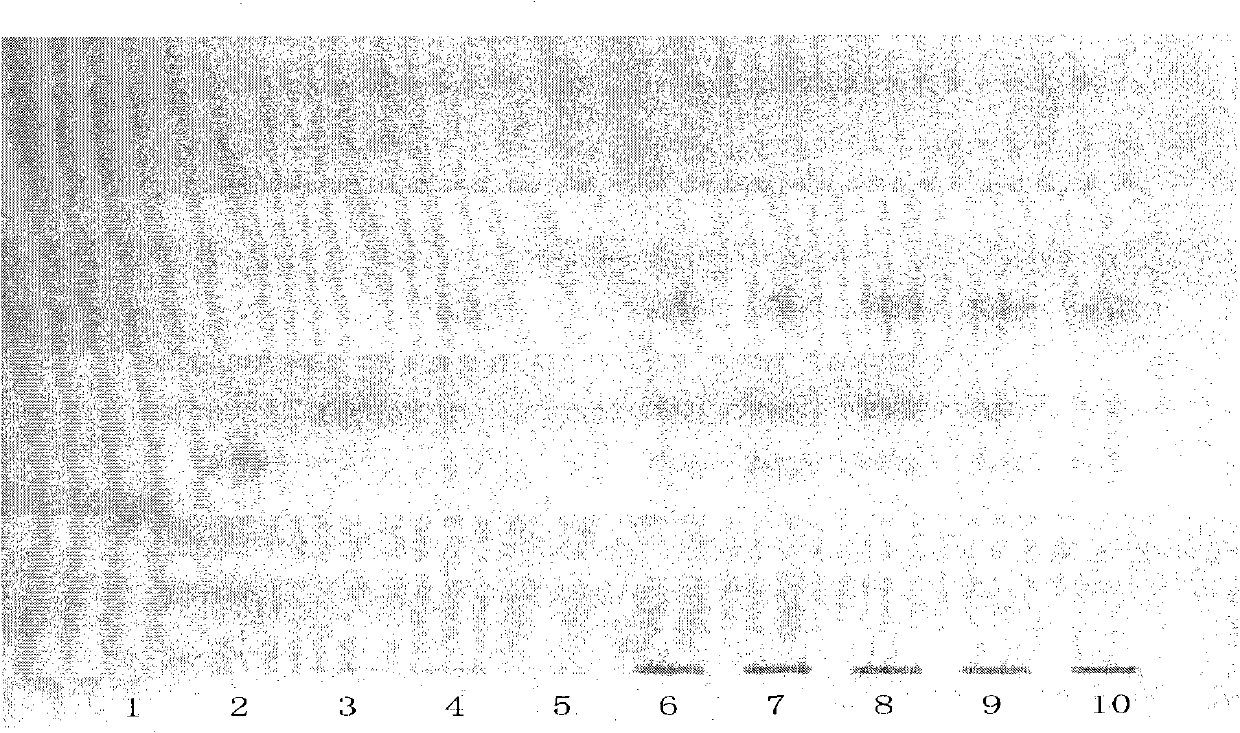
[0045] According to the specification requirements of a Compound Danshen Tablet in the Pharmacopoeia of the People's Republic of China in 2010, 2 batches of specifications (1) (5 tablets in each batch, respectively numbered 6 and 7), 2 batches of specifications (3) (5 tablets in each batch) , respectively numbered 8, 9), 1 batch of specifications (2) (2 pieces, numbered 10).
[0046] Grind the above 2 batches of specifications (1) 5 tablets per batch, 2 batches of specifications (2) 5 tablets per batch and 1 batch of specifications (3) 2 tablets with sugar coating removed, add 10ml of ether each, ultrasonicate for 5 minutes, filter After that, discard the filter residue, evaporate the filtrate to dryness, add 2ml of ethyl acetate to the residue to dissolve, and use it as the test solution respectively (the corresponding numbers are 6, 7, 8, 9, 10). Take another dihydrotanshinone Ⅰ reference substance (number 1), cryptotanshinone reference substance (number 2), tanshinone Ⅰ ref...
Embodiment 3
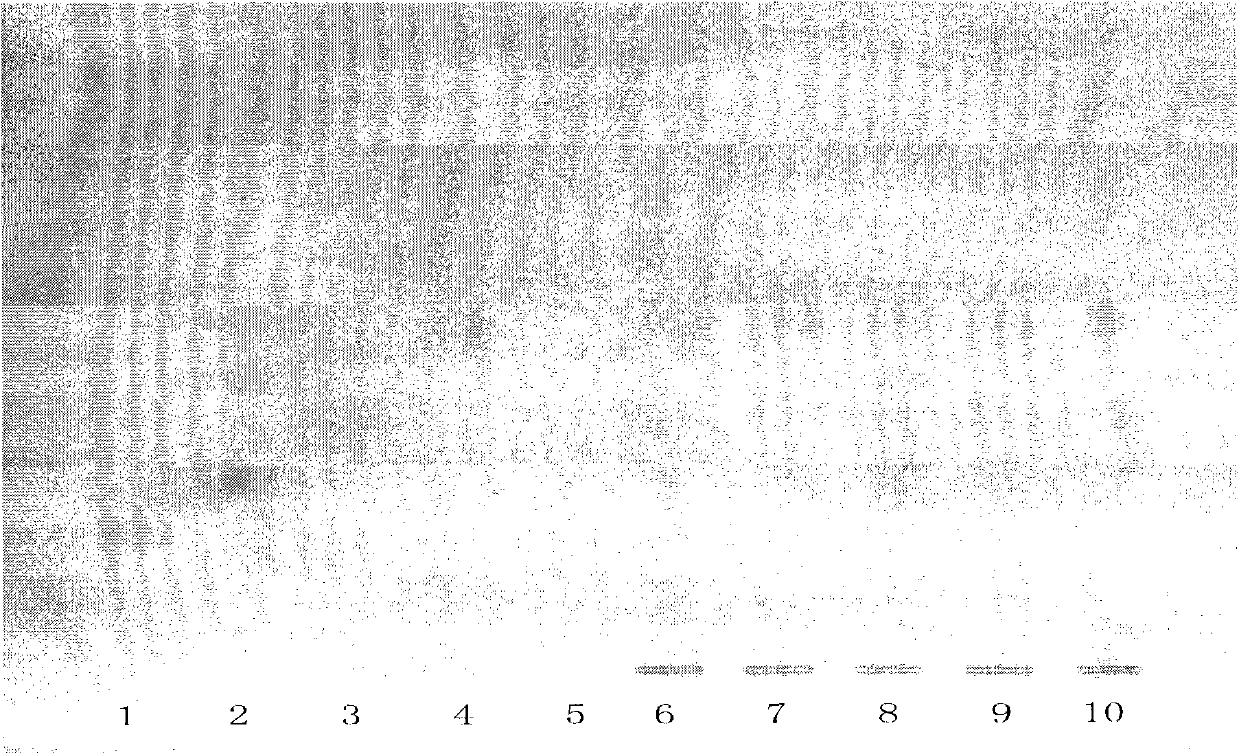
[0048] According to the specification requirements of a Compound Danshen Tablet in the Pharmacopoeia of the People's Republic of China in 2010, 2 batches of specifications (1) (5 tablets in each batch, respectively numbered 6 and 7), 2 batches of specifications (3) (5 tablets in each batch) , respectively numbered 8, 9), 1 batch of specifications (2) (2 pieces, numbered 10).
[0049] Grind the above 2 batches of specifications (1) 5 tablets per batch, 2 batches of specifications (2) 5 tablets per batch and 1 batch of specifications (3) 2 tablets with sugar coating removed, add 10ml of ether each, ultrasonicate for 5 minutes, filter After that, discard the filter residue, evaporate the filtrate to dryness, add 2ml of ethyl acetate to the residue to dissolve, and use it as the test solution respectively (the corresponding numbers are 6, 7, 8, 9, 10). Take another dihydrotanshinone Ⅰ reference substance (number 1), cryptotanshinone reference substance (number 2), tanshinone Ⅰ ref...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com