Method for testing efficacy of hog cholera lapinized virus live vaccine
A technology for swine fever rabbits and vaccines, which can be used in biochemical equipment and methods, microbial determination/inspection, measuring devices, etc., and can solve problems such as high labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
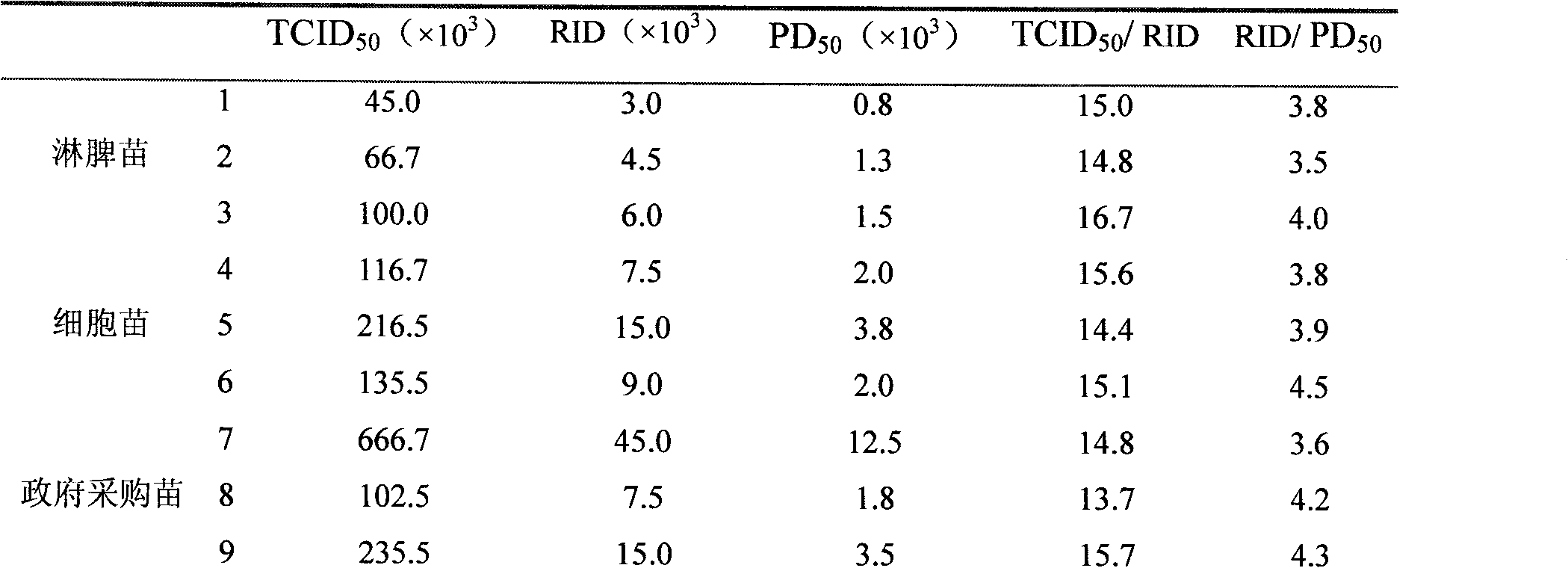
Image
Examples
Embodiment 1
[0104] 1 Reagents and materials
[0105] 1.1 Reference virus of classical swine fever
[0106] The attenuated rabbit strain of classical swine fever virus (strain C) was identified, preserved and provided by the China Veterinary Drug Administration.
[0107] 1.2 Cell lines
[0108] Rabbit kidney cells (RK-13), according to the "Procedures" detection does not contain exogenous virus.
[0109] 1.3 Cell Culture Medium
[0110] 90% MEM+10% fetal bovine serum, pH 7.2.
[0111] 1.4 Cell Maintenance Solution
[0112] 98% MEM + 2% fetal bovine serum + double antibody (final concentration 100IU / ml each), pH 7.2.
[0113] 1.5 diluent
[0114] 99% MEM + double antibody (final concentration 100IU / ml each), pH 7.2.
[0115] 1.6 Fixative
[0116] 50% acetone-methanol solution (50% acetone + 50% methanol).
[0117] 1.7 Antibodies
[0118] Fluorescent antibody against swine-derived swine fever (provided by China Veterinary Drug Administration).
[0119] 2 Determination of virus tit...
Embodiment 2
[0144] 1 Reagents and materials
[0145] 1.1 Reference virus of classical swine fever
[0146] The attenuated rabbit strain of classical swine fever virus (strain C) was identified, preserved and provided by the China Veterinary Drug Administration.
[0147] 1.2 Cell lines
[0148] Rabbit kidney cells (RK-13), according to the "Procedures" detection does not contain exogenous virus.
[0149] 1.3 Cell Culture Medium
[0150] 90% MEM+10% fetal bovine serum, pH 7.2.
[0151] 1.4 Cell Maintenance Solution
[0152] 98% MEM + 2% fetal bovine serum + double antibody (final concentration 100IU / ml each), pH 7.2.
[0153] 1.5 diluent
[0154] 99% MEM + double antibody (final concentration 100IU / ml each), pH 7.2.
[0155] 1.6 Fixative
[0156] 50% acetone-methanol solution (50% acetone + 50% methanol).
[0157] 1.7 Antibodies
[0158] Swine-derived swine fever enzyme-labeled antibody (provided by China Veterinary Drug Administration).
[0159] 1.8 DAB chromogenic solution
[...
Embodiment 3
[0189] 1 Reagents and materials
[0190] 1.1 Reference virus of classical swine fever
[0191] The attenuated rabbit strain of classical swine fever virus (strain C) was identified, preserved and provided by the China Veterinary Drug Administration.
[0192] 1.2 Cell lines
[0193] Rabbit kidney cells (RK-13), according to the "Procedures" detection does not contain exogenous virus.
[0194] 1.3 Cell Culture Medium
[0195] 90% MEM+10% fetal bovine serum, pH 7.2.
[0196] 1.4 Cell Maintenance Solution
[0197] 98% MEM + 2% fetal bovine serum + double antibody (final concentration 100IU / ml each), pH 7.2.
[0198] 1.5 diluent
[0199] 99% MEM + double antibody (final concentration 100IU / ml each), pH 7.2.
[0200] 1.6 Fixative
[0201] 50% acetone-methanol solution (50% acetone + 50% methanol).
[0202] 1.7 Antibodies
[0203] Swine-derived swine fever antibody (provided by China Veterinary Drug Administration), fluorescently labeled secondary antibody (secondary antibo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com