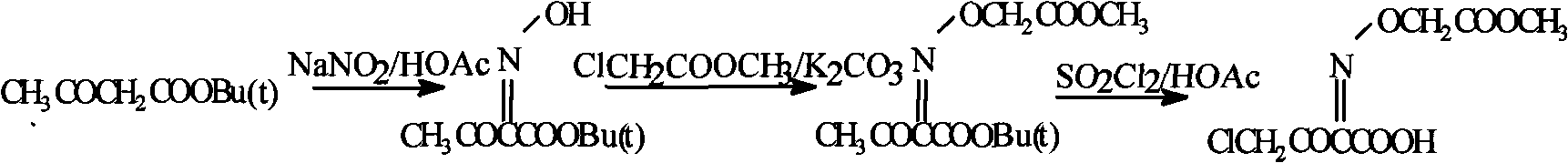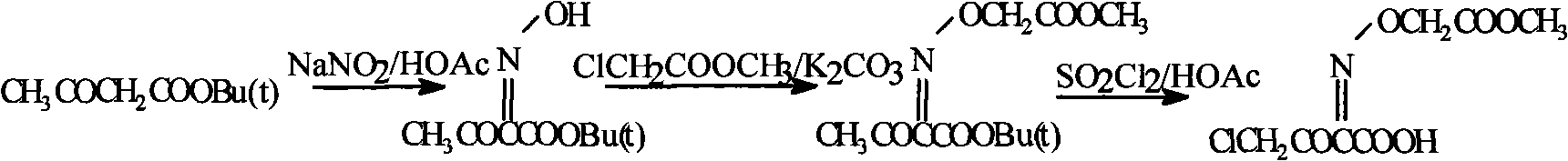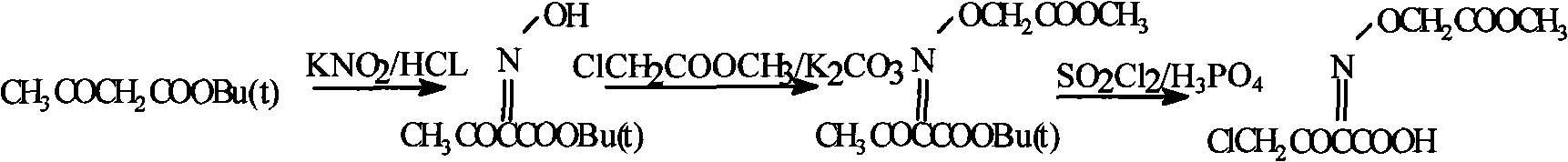Method for preparing cefixime dispersible tablet raw material intermediate
A technology of cefixime and dispersible tablets, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of high raw material cost, many wastes, and low yield, and achieve high production value, low cost, and short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Cefixime intermediate preparation method:
[0016]
[0017] Add 100g of tert-butyl acetoacetate and 100ml of glacial acetic acid to a 2000ml round bottom flask, add dropwise 100ml of water and 30g of NaNO 2 For the prepared solution, the rate of addition is controlled so that the temperature of the system does not exceed 40°C. After the addition, react at 15°C for 30 minutes. Evaporate most of the HOAC under reduced pressure, add appropriate amount of methyl chloroacetate and anhydrous K 2 CO 3 , Stir the reaction at room temperature for 10h. After the reaction is complete, add 800ml of water, add 400ml of EtOAc, separate the liquid, transfer the oil to a 1000ml flask, add 150ml of HOAc, add 200g of sulfuryl chloride dropwise at 20°C within 1h, after the drop is complete, react at this temperature for another 1h. Cool, concentrate under reduced pressure, and dry under vacuum at 50°C to obtain 36.3 g of white crystals with a measured content of 99.7%.
Embodiment 2
[0019]
[0020] Add 100g of tert-butyl acetoacetate and 100ml of 1mol / L hydrochloric acid in a 2000ml round bottom flask, add dropwise 100ml of water and 30g of KNO 2 For the prepared solution, the rate of addition is controlled so that the temperature of the system does not exceed 40°C. After the addition, react at 15°C for 30 minutes. Evaporate most of the HCl under reduced pressure, add appropriate amount of methyl chloroacetate and anhydrous K 2 CO 3 , Stir the reaction at room temperature for 10h. After the reaction, add 1000ml of water, add 400ml of EtOAc, separate the oily matter into a 1000ml flask, add 100ml of H 3 PO 4 , Add 180 g of sulfonyl chloride dropwise within 2 hours at 25°C, after the drop is complete, react at this temperature for another 2 hours. Cool, concentrate under reduced pressure, and dry under vacuum at 50°C to obtain 34.3 g of white crystals with a measured content of 101.2%.
Embodiment 3
[0022]
[0023] Add tert-butyl acetoacetate 100g, 1mol / L hydrochloric acid 100ml in the 2000ml round-bottomed flask, drop by 100ml water and 26g Ca(NO 2 ) 2 For the prepared solution, the rate of addition is controlled so that the temperature of the system does not exceed 40°C. After the addition, react at 15°C for 30 minutes. Evaporate most of HOAc under reduced pressure, add appropriate amount of methyl chloroacetate and anhydrous Na 2 CO 3 , Stir the reaction at room temperature for 8h. After the reaction is complete, add 500ml of water, add 400ml of EtOAc, separate the oil and transfer it to a 1000ml flask, add 100ml of HOAc, add 160g of sulfuryl chloride dropwise at 25°C within 2h, and then react at this temperature for another 2h. Cool, concentrate under reduced pressure, and dry under vacuum at 50°C to obtain 38.1 g of white crystals with a measured content of 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com