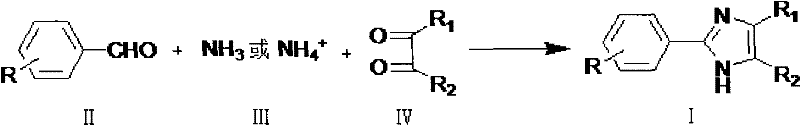
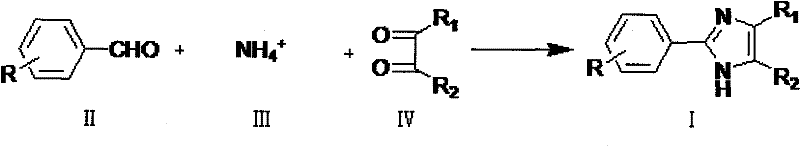
Method for preparing 2-phenylimidazole compounds
The technology of phenylimidazole and compound is applied in the field of compound preparation, can solve the problems of difficult pH value of reaction solution, severe reaction conditions, many by-products and the like, and achieves the effects of low production cost, simple operation and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In a 1000mL four-necked bottle, equipped with a thermometer, an electric stirrer, and a reflux condenser, add 200g of water, 63g of ammonium carbonate, 200g of methanol, and 46.4g of benzaldehyde, stir, heat up in a water bath, and keep the internal temperature at 35-40°C .Mix 112.5g of 28% methylglyoxal and 112g of methanol evenly, place it in a 200mL constant-pressure dropping funnel, add it dropwise to the reaction solution within 4.5 hours, and then keep the internal temperature at 35-40°C for 1 hour. Sampling for HPLC analysis It shows that the product content is 92.4184% (Area%).
[0037] After the reaction was finished, the reaction solution was concentrated to dryness under reduced pressure and methanol was recovered. 150 g of water was added to the residue, stirred evenly and cooled to crystallize, filtered, and the solid was washed twice with 25 g × 2 water, and dried to obtain 57.4 g of a yellow crude product. The yield of the crude product based on benzaldeh...
Embodiment 2
[0039] In a 500mL four-necked bottle, equipped with a thermometer, an electric stirrer, and a reflux condenser, add 30g of water, 42g of ammonium carbonate, 100g of methanol, and 46.4g of benzaldehyde, stir, heat up in a water bath, and keep the internal temperature at 35-40°C . Mix 112.5 g of 28% methylglyoxal and 60 g of methanol evenly, place it in a 200 mL constant pressure dropping funnel, and drop it into the reaction solution within 4.5 hours. When the remaining 10% of the dropwise mixed liquid was added, 8.4 g of ammonium carbonate was added. Then keep the internal temperature at 35-40° C. for 1 hour. Sample HPLC analysis showed that the product content was 94.5558% (Area%).
[0040]After the reaction, the reaction solution was concentrated to dryness under reduced pressure and methanol was recovered. Add 150g of water to the residue, stir evenly and cool down to crystallize, filter, wash the solid twice with 25g×2 water, and dry to obtain 63.4g of a yellow crude pr...
Embodiment 3
[0042] In a 1000mL four-necked bottle, equipped with a thermometer, an electric stirrer, and a reflux condenser, add 120g of water, 63g of ammonium carbonate, 100g of methanol, and 46.4g of benzaldehyde, stir, heat up in a water bath, and keep the inner temperature at 35-40°C .Mix 112.5g of 28% methylglyoxal and 112g of methanol evenly, place in a 200mL constant pressure dropping funnel, add dropwise to the reaction solution within 4.5 hours, and then keep the internal temperature at 35-40°C for 8 hours.
[0043] After the reaction was finished, the reaction solution was concentrated to dryness under reduced pressure and methanol was recovered. Add 150 g of water to the residue, stir evenly and cool down to crystallize, filter, wash the solid twice with 25 g×2 water, and obtain 60.4 g of a yellow crude product after drying. The yield of the crude product based on benzaldehyde was 87.32%. The analysis result of the crude product was 96.8605% (Area%).
[0044] Using self-made wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com