Clean preparation method for basic magnesium carbonate
A magnesium carbonate, basic technology, applied in magnesium carbonate and other directions, can solve the problems of narrow application range, waste of mineral resources, low added value of products, etc., to achieve the effect of protecting the environment and saving water resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Mix 46.7g of natural mineral brucite with a particle size of about 400 μm and 731.6 g of deionized water to obtain 0.8 L of brucite slurry with a solid content of 6%, grind it with a colloid mill for 20 minutes to make the particle size less than 45 μm, and then place In the high-pressure reactor, 0.35Mpa of CO 2 Under pressure, heat up to 170°C under stirring conditions, react for 5 hours, stop ventilation and heating, centrifuge the obtained product after cooling, and directly dry at 50°C for 10 hours to obtain basic magnesium carbonate with a particle size of micron.
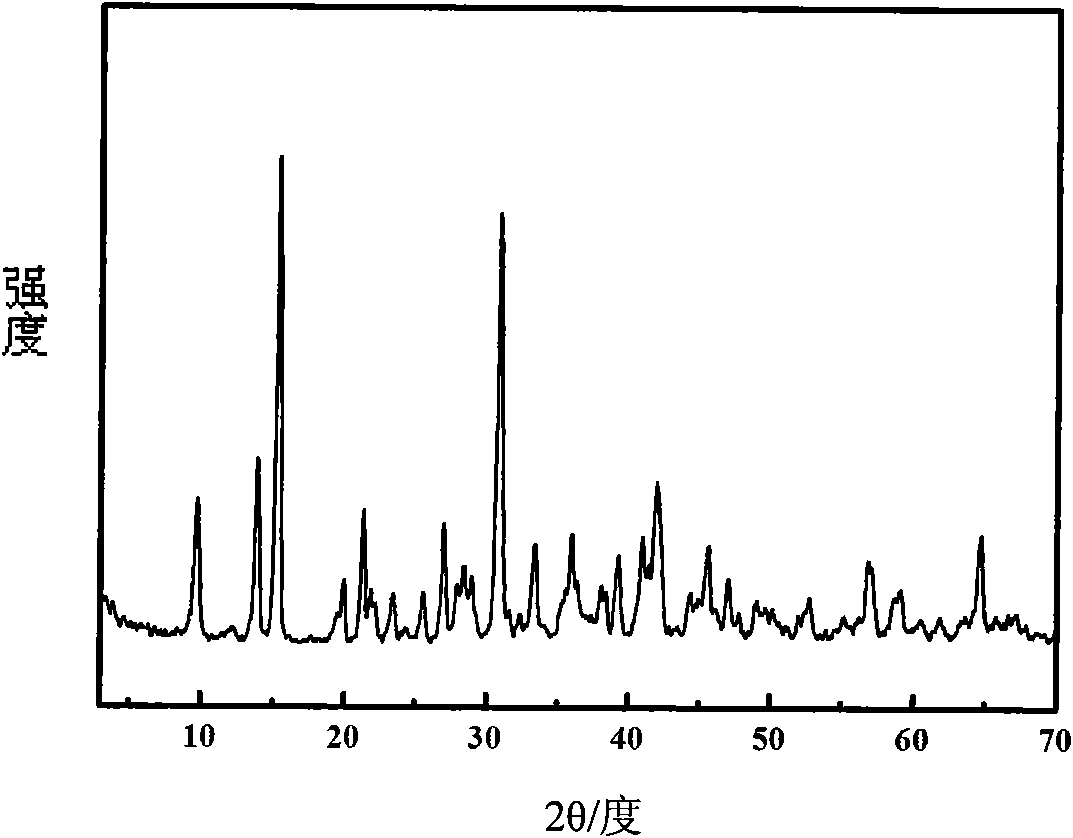
[0023] The crystal structure of the sample was characterized by XRD-6000 X-ray powder diffractometer from Shimadzu Corporation, Japan. figure 1 It is the XRD spectrogram of the sample gained in Example 1. As can be seen from the figure, the characteristic peaks in the standard magnesium basic carbonate XRD spectrogram include 2θ=9.6 °, 13.8 °, 15.2 °, and 30.8 °. There is a sharp peak shape of each ch...
Embodiment 2
[0025] Mix 46.7g of natural mineral brucite with a particle size of about 200μm and 731.6g of deionized water to obtain 0.8L of brucite slurry with a solid content of 6%, grind it with a ball mill for 15min to make the particle size less than 10μm, and then place In the high-pressure reactor, 0.78Mpa of CO 2 Under pressure, heat up to 100°C under stirring conditions, react for 3 hours, stop ventilation and heating, centrifuge the obtained product after cooling, and directly dry at 160°C for 2 hours to obtain basic magnesium carbonate with a particle size of micron.
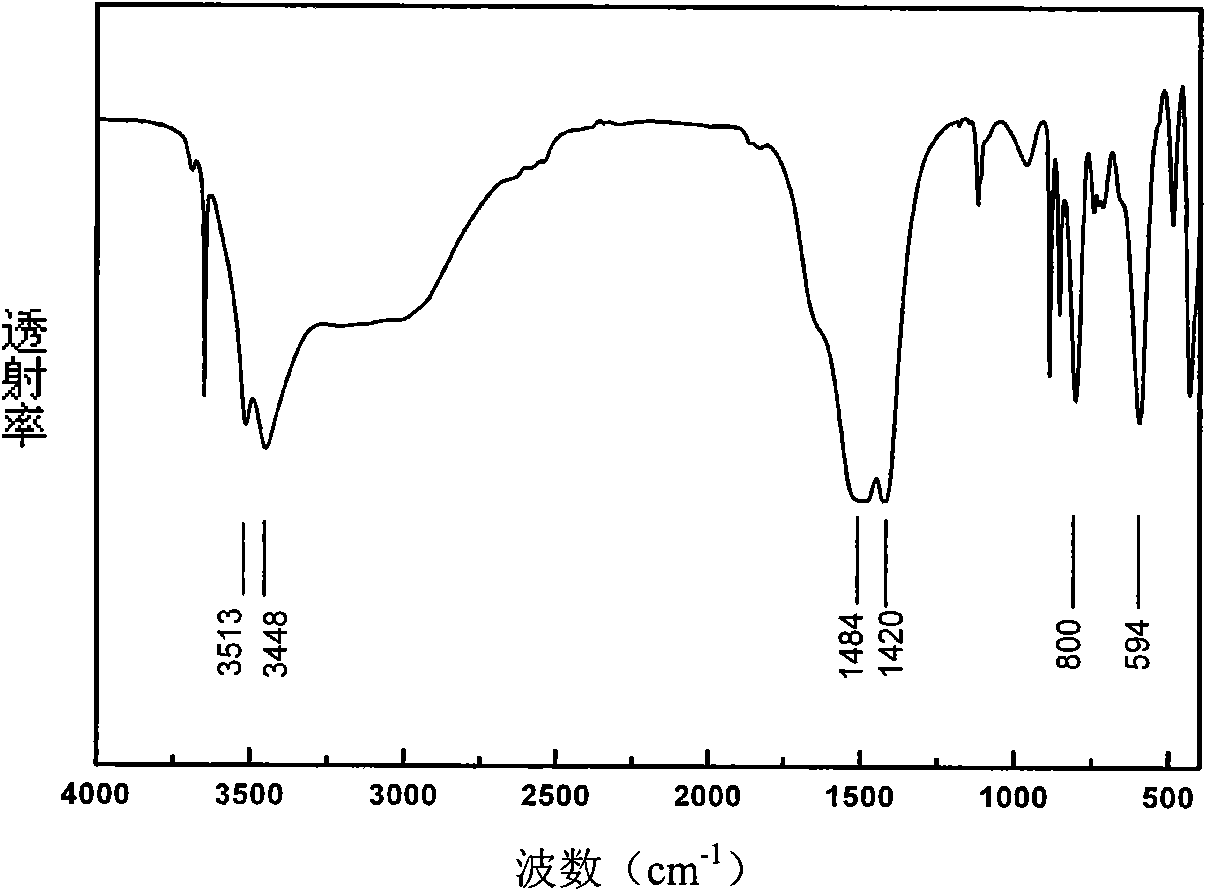
[0026] The samples were qualitatively analyzed by a Vector22 Fourier transform infrared spectrometer from Bruker, Germany. The Fourier transform spectrum (FT-IR) spectrogram of sample obtained by embodiment 2 is as follows figure 2 shown, of which 3450cm -1 and nearby 3513cm -1 The vibration peak is caused by H 2 O molecule and OH - Caused by stretching and bending vibrations of ions, indicating that the pro...
Embodiment 3
[0032] The synthetic magnesium hydroxide of 24.5g particle diameter about 10 μm is mixed with 790.4g deionized water, obtains 0.8L solid content and is the magnesium hydroxide slurry of 3%, grinds 15min with colloid mill, makes its particle diameter less than 0.5 μm, then Placed in a high-pressure reactor and fed with 0.35Mpa of CO 2 Under pressure, heat up to 60°C under stirring conditions, react for 5 hours, stop ventilation and heating, and after cooling, centrifuge the obtained product, and directly dry at 80°C for 8 hours to obtain basic magnesium carbonate with a particle size of micron.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com