Preparation for promoting revascularization or angiogenesis and preparation method thereof
A kind of preparation, a new technology, applied in the direction of cardiovascular system diseases, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of the actual efficiency of surviving cells with low survival rate, and limit the promotion and application of stem cell therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Preparation of monocytes.
[0032] 100 mL of blood was added to the density gradient agent Histopaque-1077 (Sigma), and 30 mL of blood was added per 15 mL of Histopaque. Centrifuge the test tube containing the blood and the gradient reagent at 400G for 30 minutes at room temperature. After stratification, use a sterile disposable needle to draw the middle opaque layer, which is the suspension rich in mononuclear cells. Depending on the situation, every 100ml of blood can get 1x10 8 ~1x10 10 monocytes.
Embodiment 2
[0033] Example 2. Acquisition of EPC cells.
[0034] For the acquisition method of type I EPC cells: the mononuclear cell-rich suspension was added with 10% human serum albumin and 1% growth factor additive EGM (purchased by Swiss Lonza Company, which contains vascular endothelial growth factor VEGF-1, basic fibroblast growth factor FGF-2, epidermal growth factor EGF, and insulin-like growth factor IGF-1) in EBM-2 medium at 1×10 per square centimeter 6 The density of the cells was cultured for 4 days, and the adherent cells were digested with trypsin and collected at 2×10 per square centimeter. 5 density for 2 days. The obtained type I EPCs are spindle-shaped cells with typical endothelial cell characteristics of endocytosing acetylated-LDL and binding UEA-1 lectin, and expressing a large amount of vascular endothelial cells Cell growth factor receptors KDR, CD31, CD14, CD45, a small amount of expression of CD34, CD133 and VE-cadherin.
[0035] For the acquisition method of...
Embodiment 3
[0038] Example 3. Acquisition of pro-angiogenic or neoplastic agents.
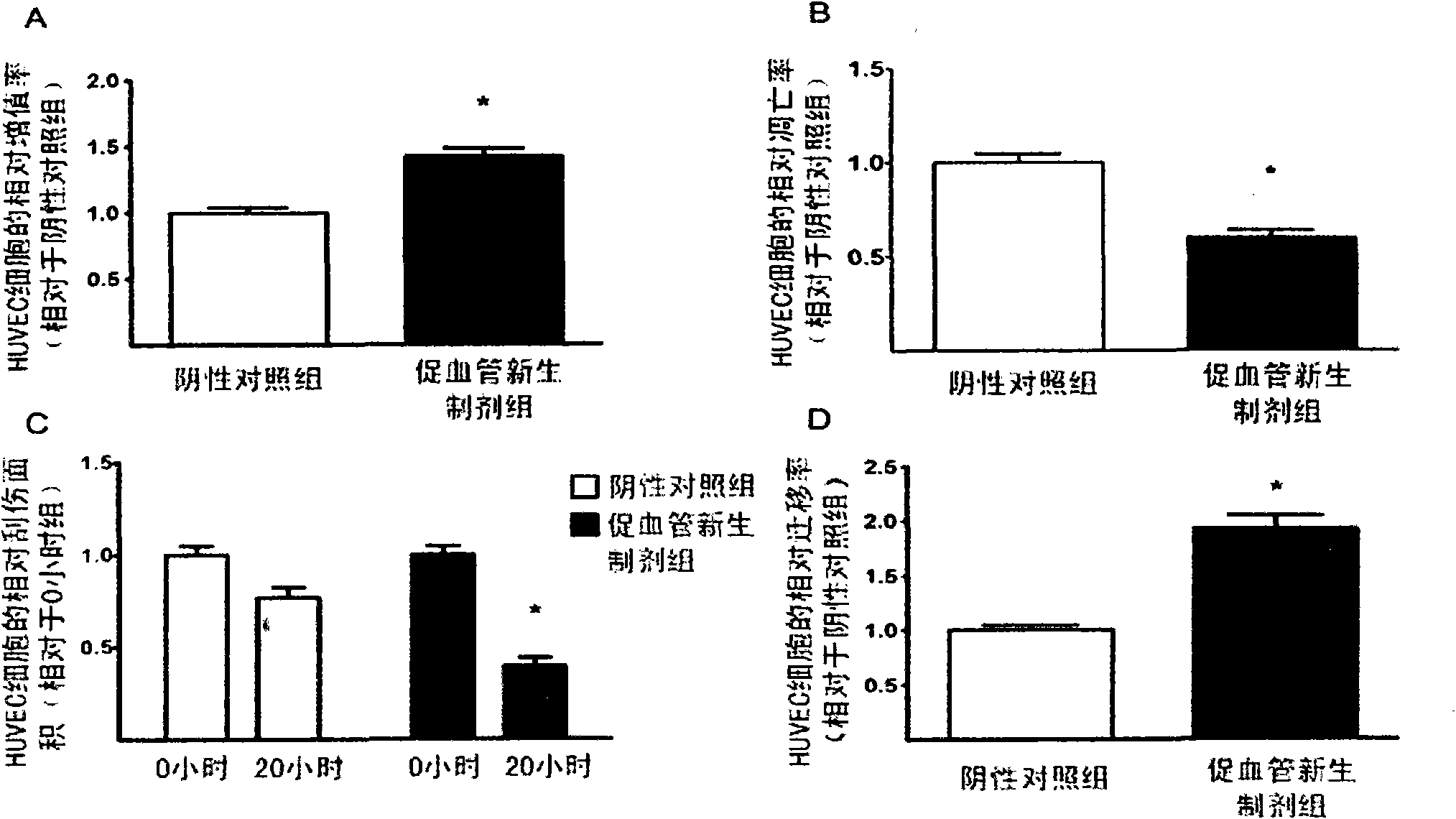
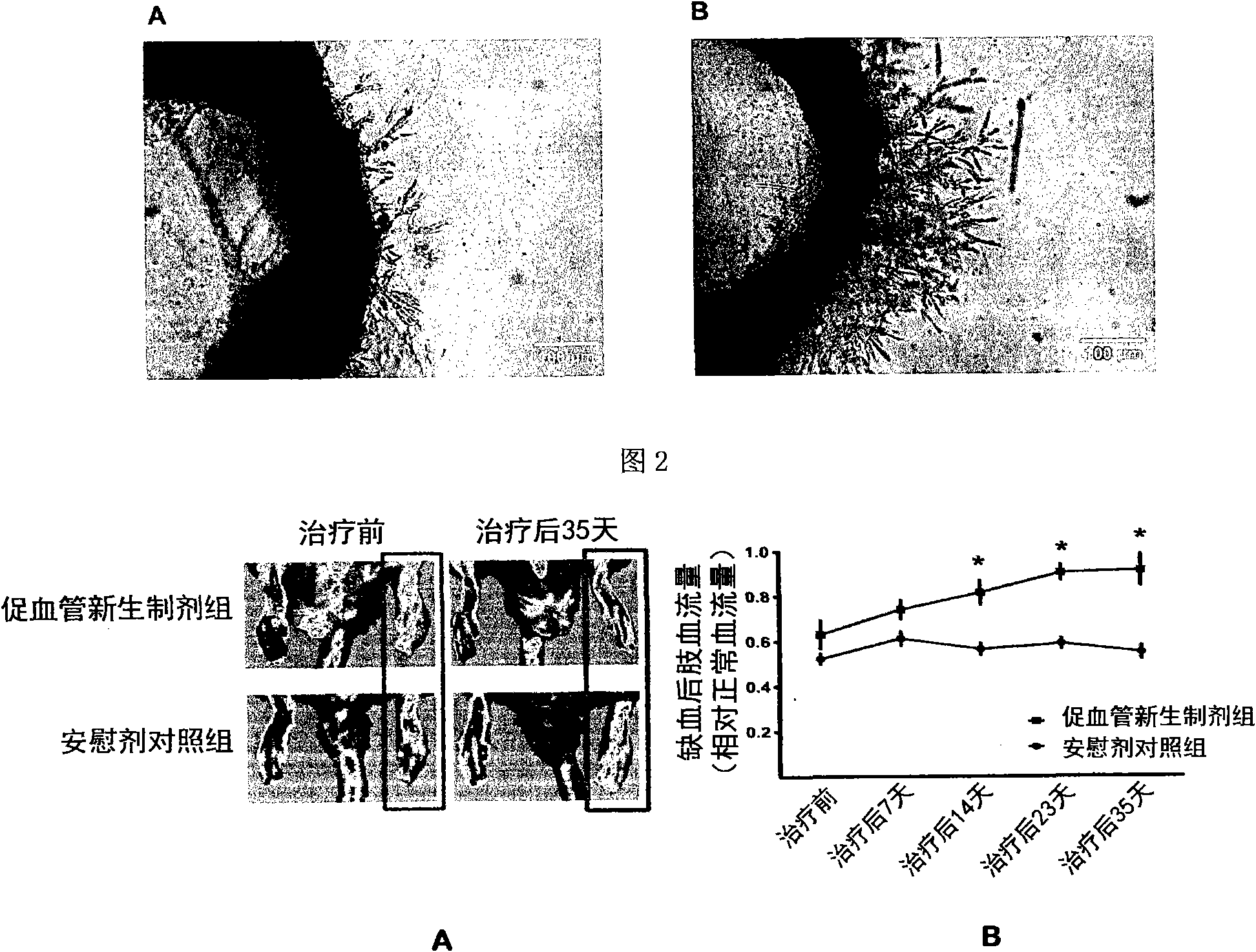
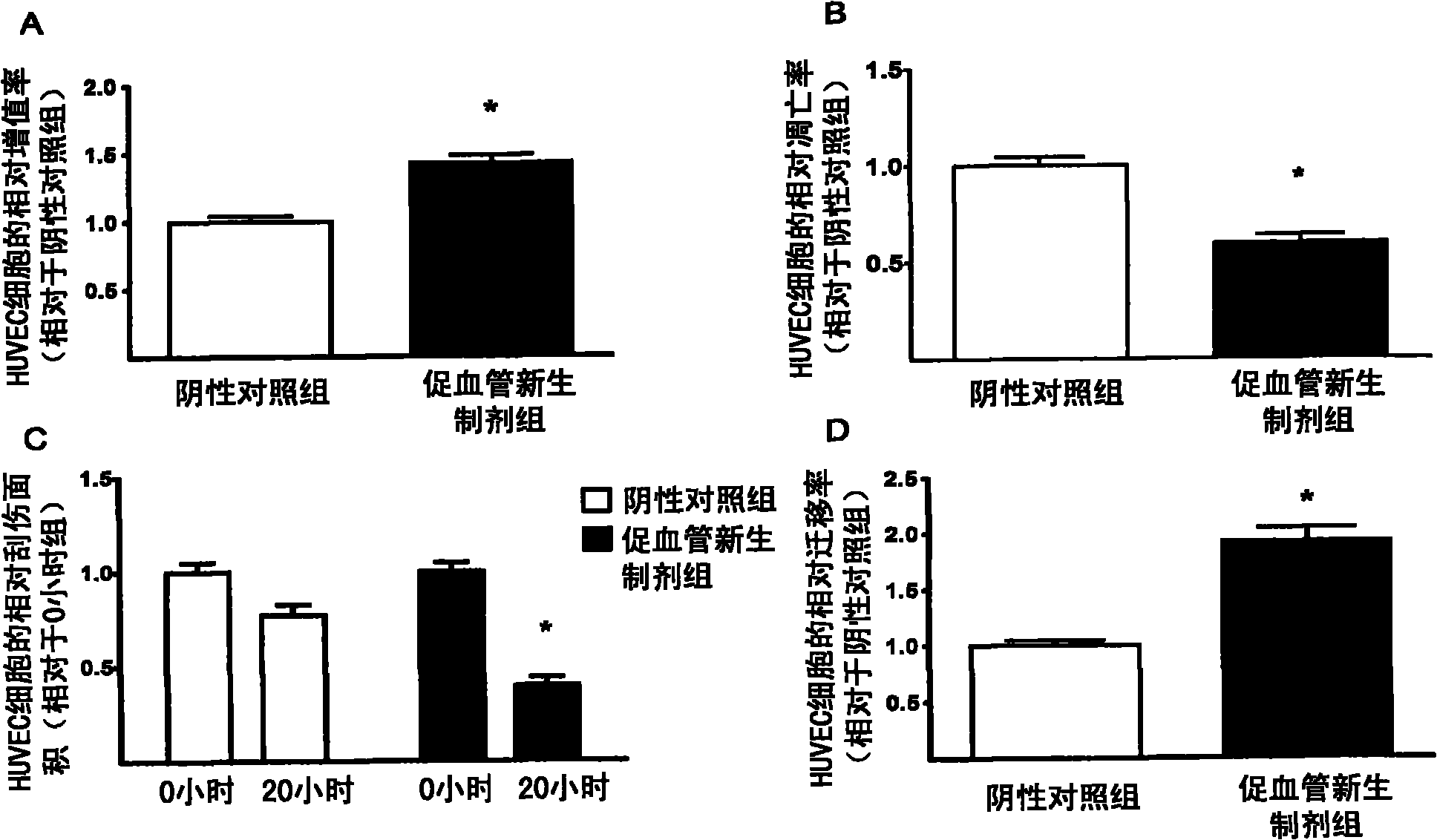
[0039] The type I EPC cells obtained in Example 2 were placed in phosphate buffered saline (PBS) without any growth factors (2×10 per square centimeter). 5 cells), cultured in an environment with an oxygen concentration of 1.5% for 2 days, and supplemented with 1% medical human serum albumin. After the culture was completed, the cell-free medium rich in cell growth factors was collected, and the adherent EPC cells were discarded. The collected cell-free medium was passed through a filter with a pore size of 0.2 microns to remove cell impurities and debris, and then stored in a -80°C freezer for later use. As the case may be, every 1x10 6 Each EPC cell can prepare 2-5 ml of the angiogenic regeneration or neonatal preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com