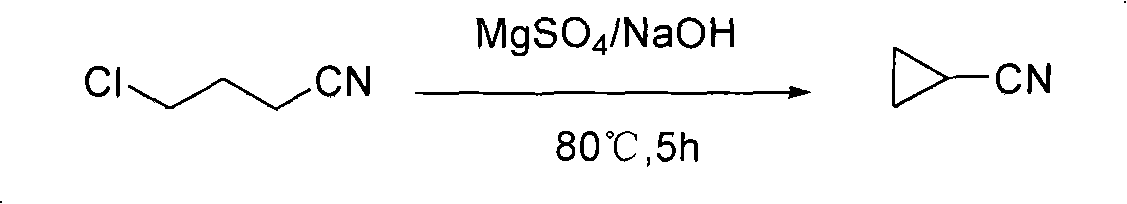Method for artificially synthesizing cyclopropanecarbonitrile
The technology of a cyclopropyl nitrile and a synthesis method, which is applied in the field of medicine and chemical industry, can solve the problems of low water content of the product, low yield, equipment destruction yield and the like, and achieves that the temperature control method is simple and easy to operate, the reaction process is gentle, and the separation The effect of purification convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of cyclopropylnitrile
[0018] In a 250ml four-neck flask equipped with a mechanical stirrer, a thermometer, a reflux condenser, and a constant pressure dropping funnel, put dimethyl sulfoxide (45ml), anhydrous magnesium sulfate (9.0g), and sodium hydroxide (26.4g) , control the temperature at 60-85° C., and drop 4-chlorobutyronitrile (53.5 g) into the reaction system within 1 hour by means of dropwise addition. After the dropwise addition, it was cooled down to room temperature naturally, and reacted at 80° C. for 5 hours. Cool, and slowly inject 100ml of water into the water bath at room temperature, then slowly add concentrated hydrochloric acid to neutralize the reaction solution, adjust the pH=6.5, and finally adopt the azeotropic distillation method, use the constant pressure dropping funnel to continuously circulate and separate the oil and water, and then obtain the free The color oil was 34.0 g, the yield was 98.0%, and the purity was 99.7%.
Embodiment 2
[0020] Preparation of cyclopropylnitrile
[0021] In a 250ml four-neck flask equipped with a mechanical stirrer, a thermometer, a reflux condenser, and a constant pressure dropping funnel, put dimethyl sulfoxide (45ml), anhydrous sodium sulfate (8.9g), and sodium hydroxide (26.4g) , under the condition of 60-85° C., 4-chlorobutyronitrile (53.5 g) was added dropwise to the reaction system within 1 hour by means of dropwise addition. After the dropwise addition, it was cooled down to room temperature naturally, and reacted at 80° C. for 5 hours. Cool, slowly inject 100ml of water into the water bath at room temperature, then slowly add concentrated hydrochloric acid to neutralize the reaction solution, adjust the pH=6.8, and finally adopt the azeotropic distillation method, and use the constant pressure dropping funnel to continuously circulate and separate the oil and water. The color oil was 33.1 g, the yield was 95.0%, and the purity was 99.3%.
Embodiment 4
[0023] Preparation of cyclopropylnitrile
[0024] In a 250ml four-neck flask equipped with a mechanical stirrer, a thermometer, a reflux condenser, and a constant pressure dropping funnel, put N, N dimethylformyl (45ml), anhydrous magnesium sulfate (9.0g), sodium hydroxide (26.4g), at 60-85°C, dropwise added 4-chlorobutyronitrile (53.5g) into the reaction system within 1 hour. After the dropwise addition, it was cooled down to room temperature naturally, and reacted at 100° C. for 5 hours. Cool, slowly inject 100ml of water into the water bath at room temperature, then slowly add concentrated hydrochloric acid to neutralize the reaction solution, adjust the pH=6.8, and finally adopt the azeotropic distillation method, and use the constant pressure dropping funnel to continuously circulate and separate the oil and water. 32 g of colored oil, yield 92%, purity 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com