Application of beta-interferon to medicament making
A technology for interferon and use, which is applied in the field of medical use of beta-interferon, and can solve problems such as toxic side effects and clinical treatment limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
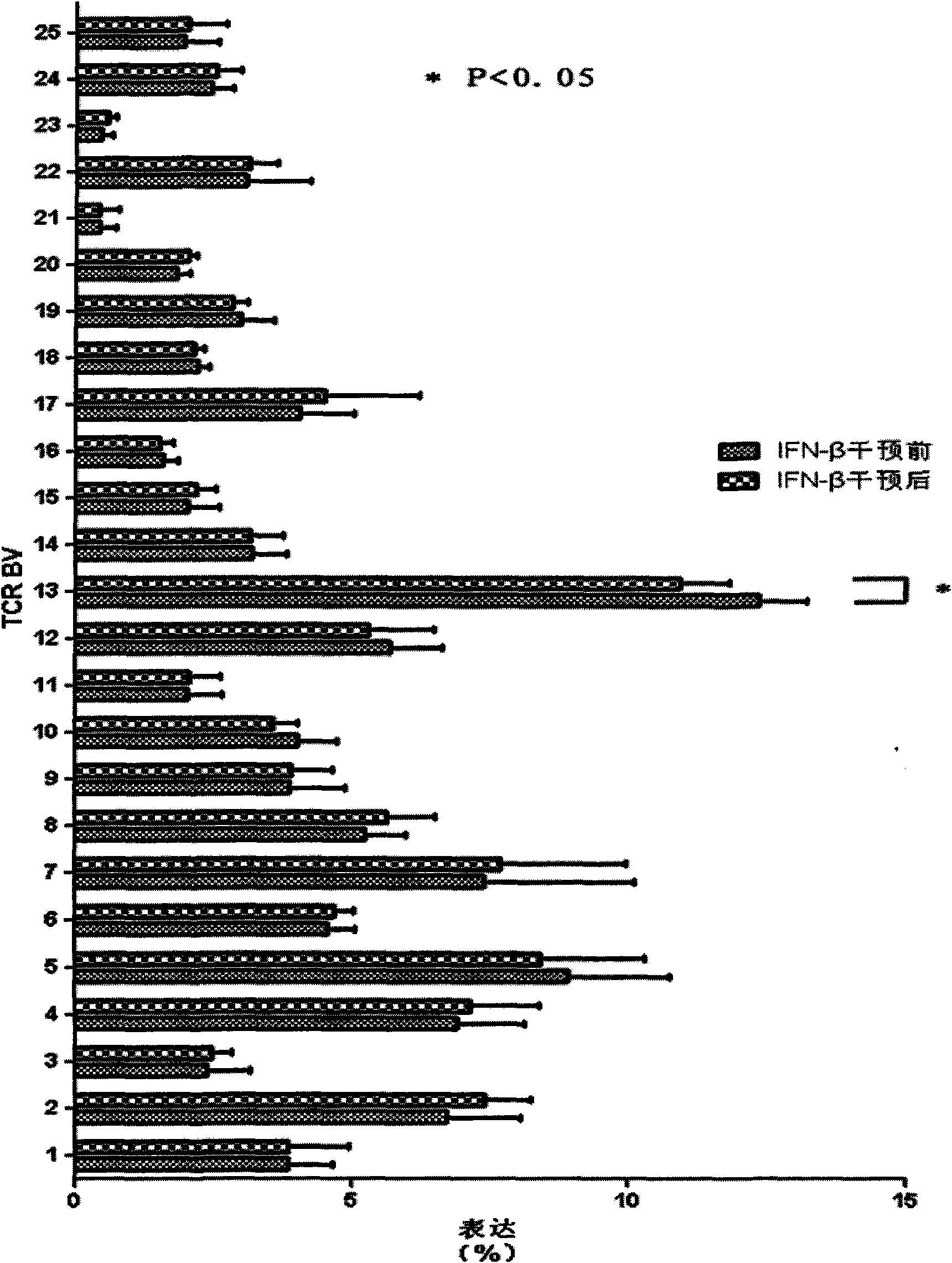
[0063] Embodiment 1 Quantitative PCR detects TCR BV
[0064] The RNA of peripheral blood mononuclear cells was extracted according to the operating instructions of Qiagen Company RNeasy Mini Kit; then according to TAKARA Company PrimeScript TM Operating instructions of the RNA reverse transcription kit Reverse transcribe the obtained RNA to obtain cDNA.
[0065] Before and after IFN-β intervention, the cDNA of peripheral blood mononuclear cells of RA patients was analyzed by quantitative PCR. It was found that before and after the intervention, although TCR BV13 was predominantly expressed, the expression of TCR BV13 (Pfigure 1 ).
Embodiment 2I
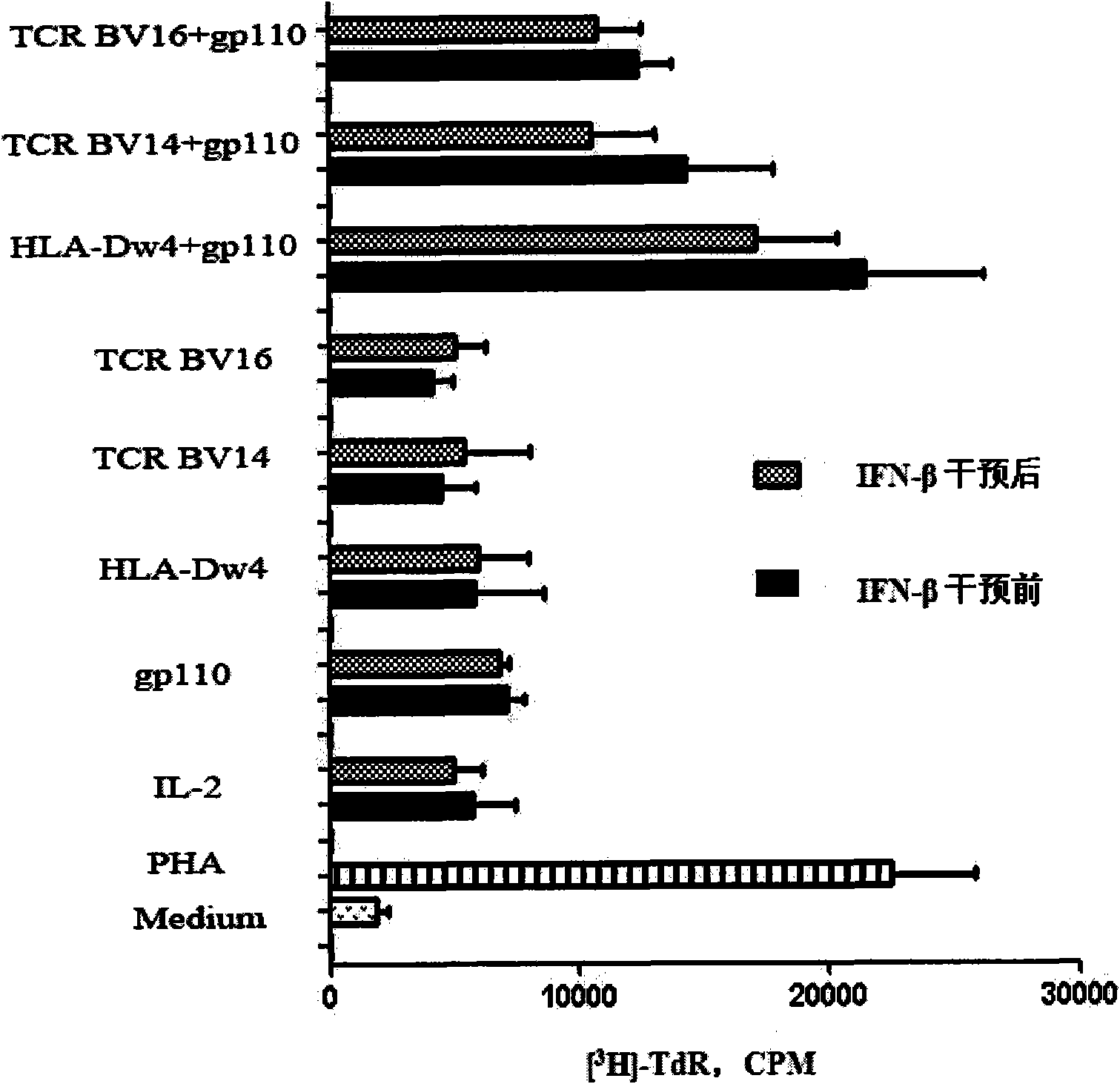
[0066] Example 2 CD3 before and after IFN-β intervention + Changes in T cell proliferation
[0067] The various conjugated peptides were synthesized by Jill Biochemical (Shanghai) Co., Ltd., with a purity greater than 95%.
[0068] CD3 + T cells were seeded in a 96-well round-bottom plate at a density of 1×10 5 cells / well, the non-CD3 cells after sorting by magnetic beads + Irradiated T cells, as APC (CD3 + T:APC=2:1). Various synthetic coupling peptides (see Table 2) were added to the culture system at a final concentration of 40 μg / ml, and a small amount of IL-2 was added. Place at 37 °C 5% CO 2 Incubator for 7 days. 16-18 hours before antigenic peptide stimulation for 7 days, add 1 μCi of [ 3 H]-TdR. Cells were collected and detected by a β liquid scintillation instrument[ 3 CPM values for H]-TdR incorporation.
[0069] Experiments have proved that the conjugated peptide with HLA-Dw4 / EBV functional domain can significantly induce CD3 in RA patients + The role ...
Embodiment 3I
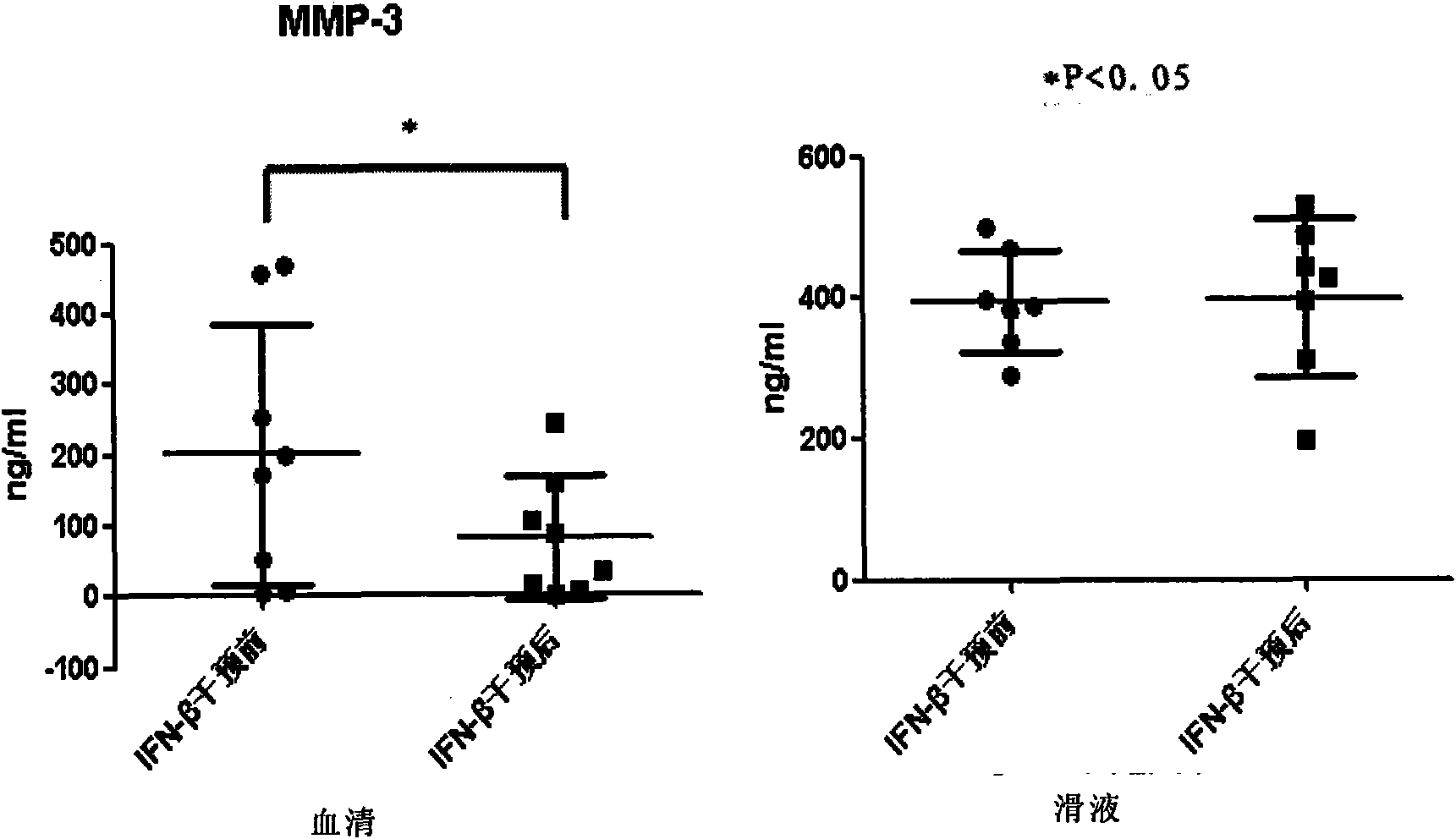
[0070] Changes of cytokines before and after the intervention of embodiment 3 IFN-β
[0071] RA intervention group: Before IFN-β intervention, three cytokines in serum and synovial fluid were detected, including IL-17 mainly secreted by Th17 cells; negative regulator IL-10; and inflammatory cells secreted by Th1 cells Factor IFN-γ. One week after IFN-β intervention, IL-17, IL-10 and IFN-γ in serum and synovial fluid were detected again. The results of the analysis and comparison are shown in Table 3.
[0072] In the experiment, according to the corresponding kit, with the concentration as the abscissa and the measured average value as the ordinate, the standard curves of IL-177, IL-10 and IFN-γ are respectively: Y=0.032+0.001X, Y=0.155 +0.007X and Y=-0.024+0.001X.
[0073] Table 3 Change trend of IL-17, IL-10 and IFN-γ in serum and synovial fluid of RA patients
[0074]
[0075] Note: ↑ indicates the level of cytokines after IFN-β intervention, which is higher than that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com