Azo and diaza derivatives and uses thereof in phototherapy
A compound, stand-alone technology, applied in the field of azo and diazo derivatives and their use in phototherapy, able to solve the problem of dyes not preferentially localizing malignant tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Example 1: Compounds for Phototherapy
[0179] 1.a Type 1 phototherapeutic agent
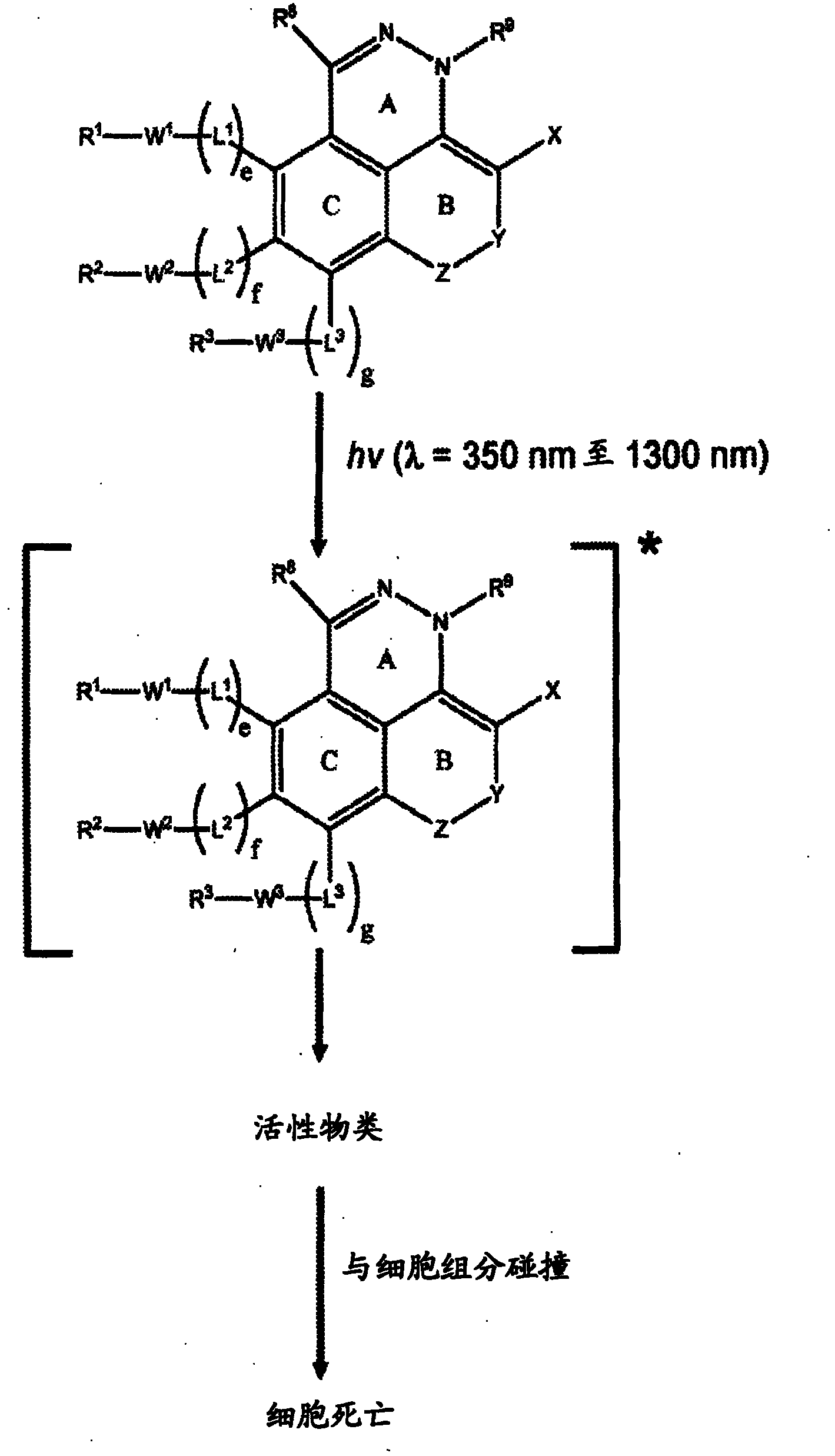
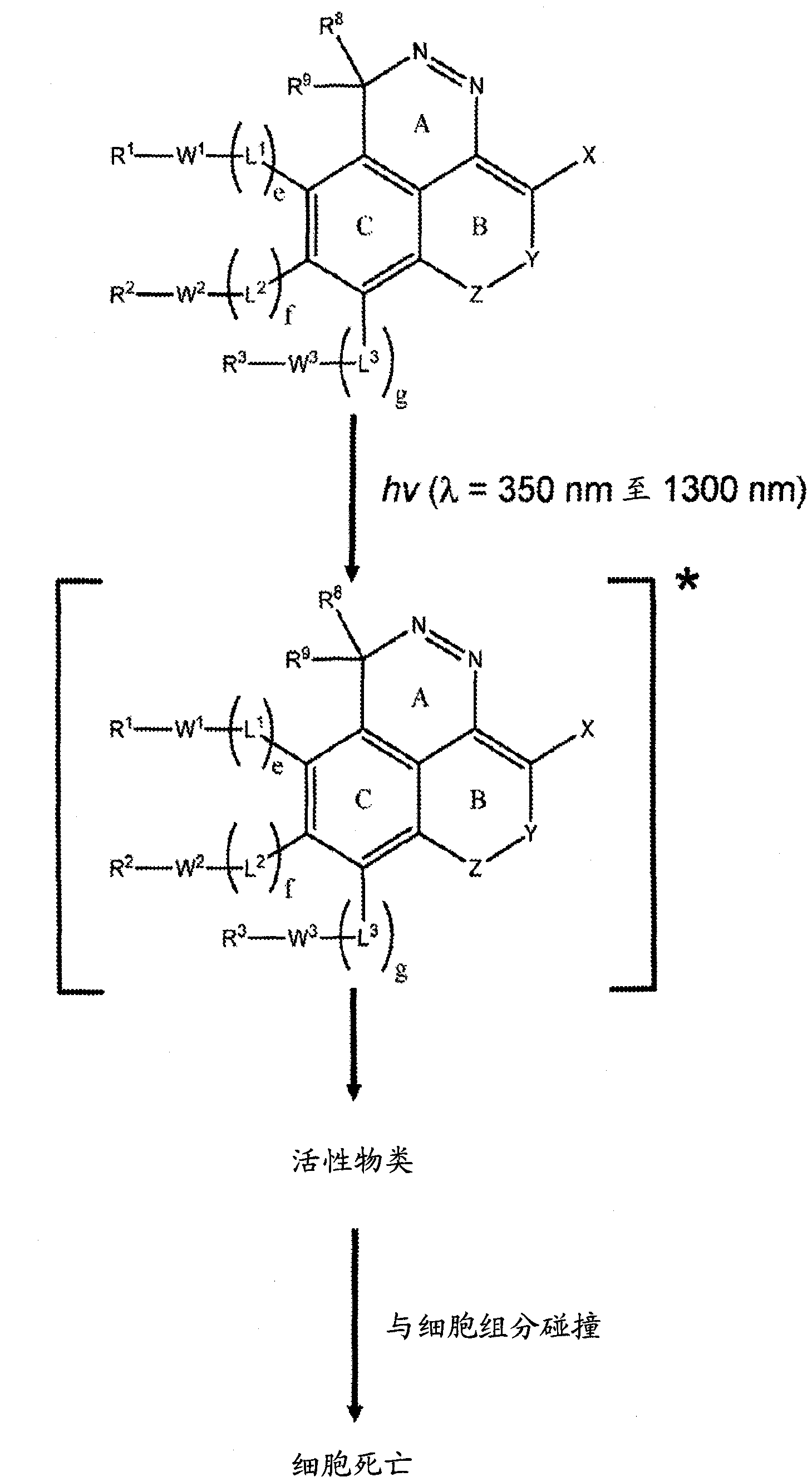
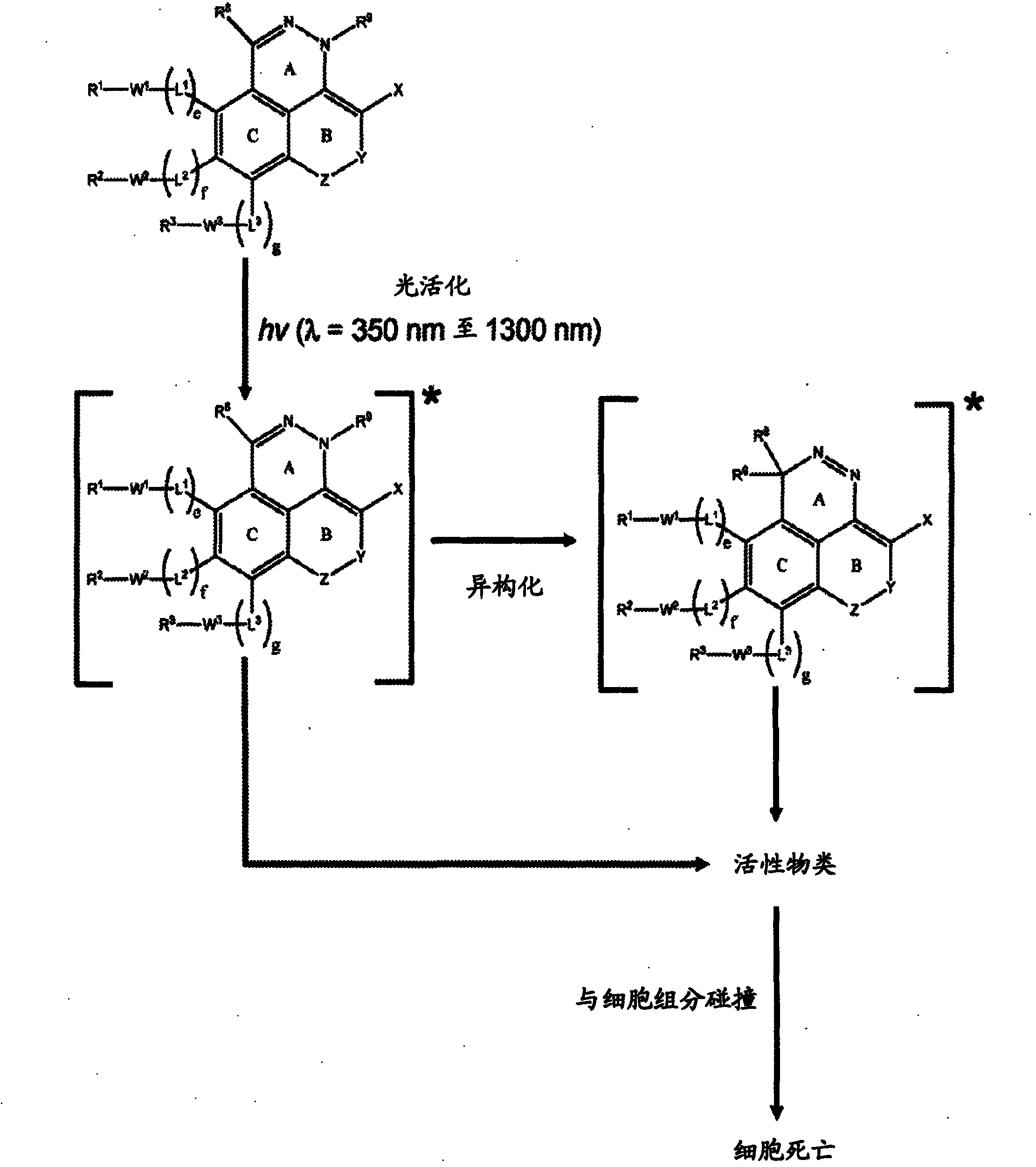
[0180] The present invention provides Type 1 phototherapeutic agents (including compositions, articles, and formulations) and methods of using and preparing Type 1 phototherapeutic agents. Type 1 phototherapeutic agents of the present invention include compounds comprising a first ring A, a second unsaturated ring B, and a third aromatic ring having an intracyclic azo or intracyclic diazo group, so The rings are in a condensed ring configuration in which ring A is fused to both unsaturated ring B and aromatic ring C, and in which unsaturated ring B and aromatic ring C are also fused to each other. The incorporation of unsaturated ring B in a fused-ring configuration in some compounds can enhance stability prior to photoactivation and can extend the conjugation of the fused-ring azo or diazo compounds, allowing light to Activation and internal energy transfer processes are carried out,...
Embodiment 1b
[0242] Example 1.b(iv): Phototherapy and Cell Viability Measurements
[0243] A general procedure was performed for measuring cell viability upon exposure of tumor cells to a fused ring diazo compound of formula (FX33) and electromagnetic radiation. The compound of formula (FX33) has an absorption maximum (λ max ). Cell viability was measured by the standard WST-1 assay using the human myelogenous leukemia U937 cell line. In this operation, U397 leukemia cells (0.5×10 6 ) were plated on standard T-25 cell culture dishes and exposed to 4 control conditions and a series of experimental conditions corresponding to a series of fused ring diazo compound concentrations, summarized in Table 1.
[0244] Table 1: Control and experimental conditions for cell viability measurements
[0245] Control 1
No electromagnetic radiation, no photosensitizer
Control 2
Electromagnetic radiation, no photosensitizers
Control 3
No electromagnetic radiation, pho...
Embodiment 2
[0250] Example 2: Light Therapy
[0251] Phototherapy, such as photodynamic therapy (PDT), typically employs a photosensitizer (PS) in combination with visible or near-infrared electromagnetic radiation to produce active species that kill or otherwise degrade target cells, such as tumors or other lesions . The present invention provides phototherapeutic agents useful in phototherapy.
[0252] The present invention includes phototherapy wherein a phototherapeutic agent comprising a compound of any of Formulas (FX1)-(FX40) is administered to a patient, eg, wherein a therapeutically effective amount of such a component is administered to a patient in need of treatment. In some embodiments, compounds of the invention provide photoreagents that can be selectively targeted and delivered to target tissues such as tumors, sites of inflammation, or other lesions. Upon administration, the phototherapeutic agent is optionally allowed to accumulate in a target area of interest (eg, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com