Metal nanoshells for biosensing applications
a biosensing and metal nanotechnology, applied in the field of particles, can solve the problems of sensitivity decline, poor reproducibility, and difficulty in substrate preparation of sers enhancement methods of near infrared ft-raman spectroscopy, and achieve the effects of reducing the sensitivity of the ser enhancement method, and improving the reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Surface Enhanced Raman Scattering (SERS) Using Metal Nanoshells
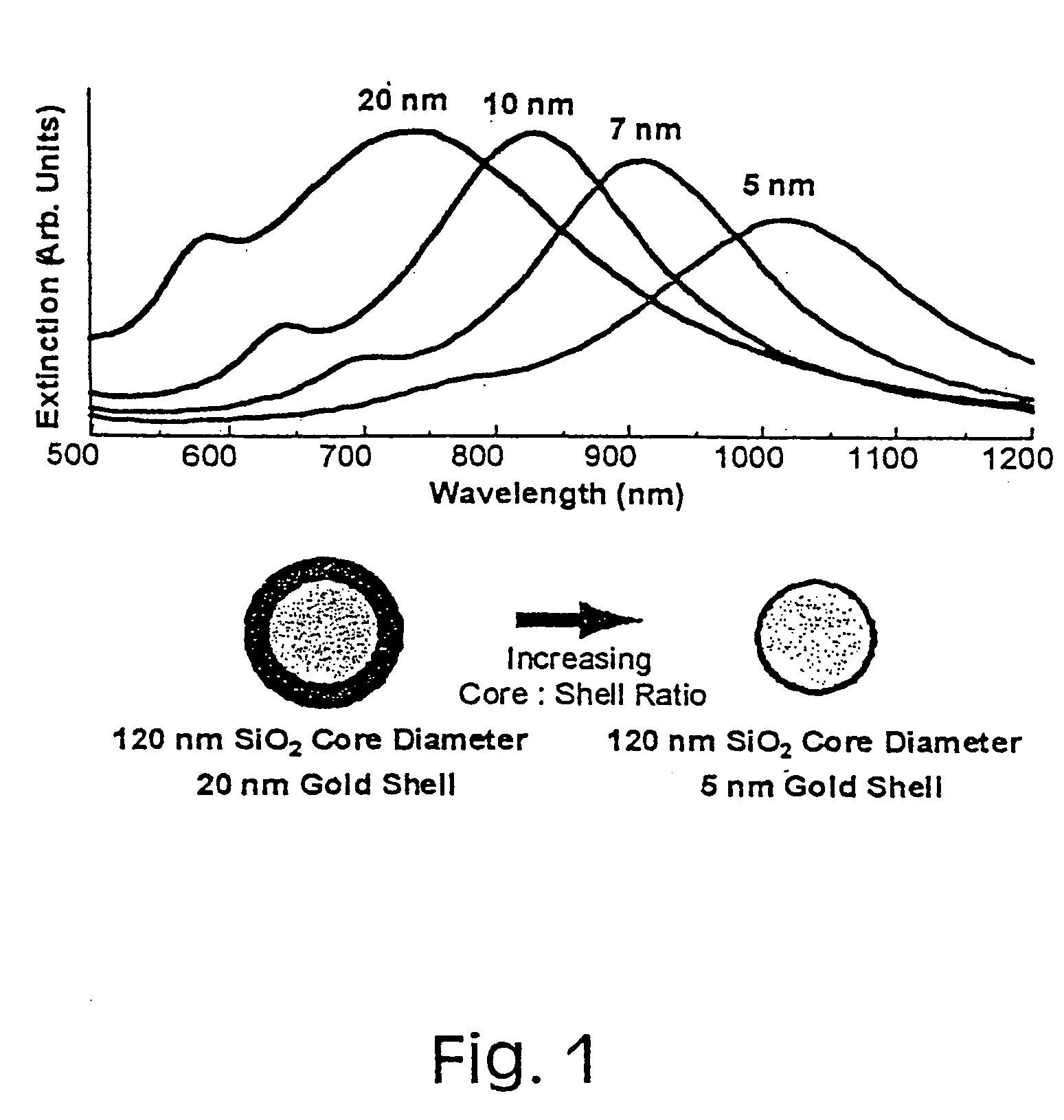
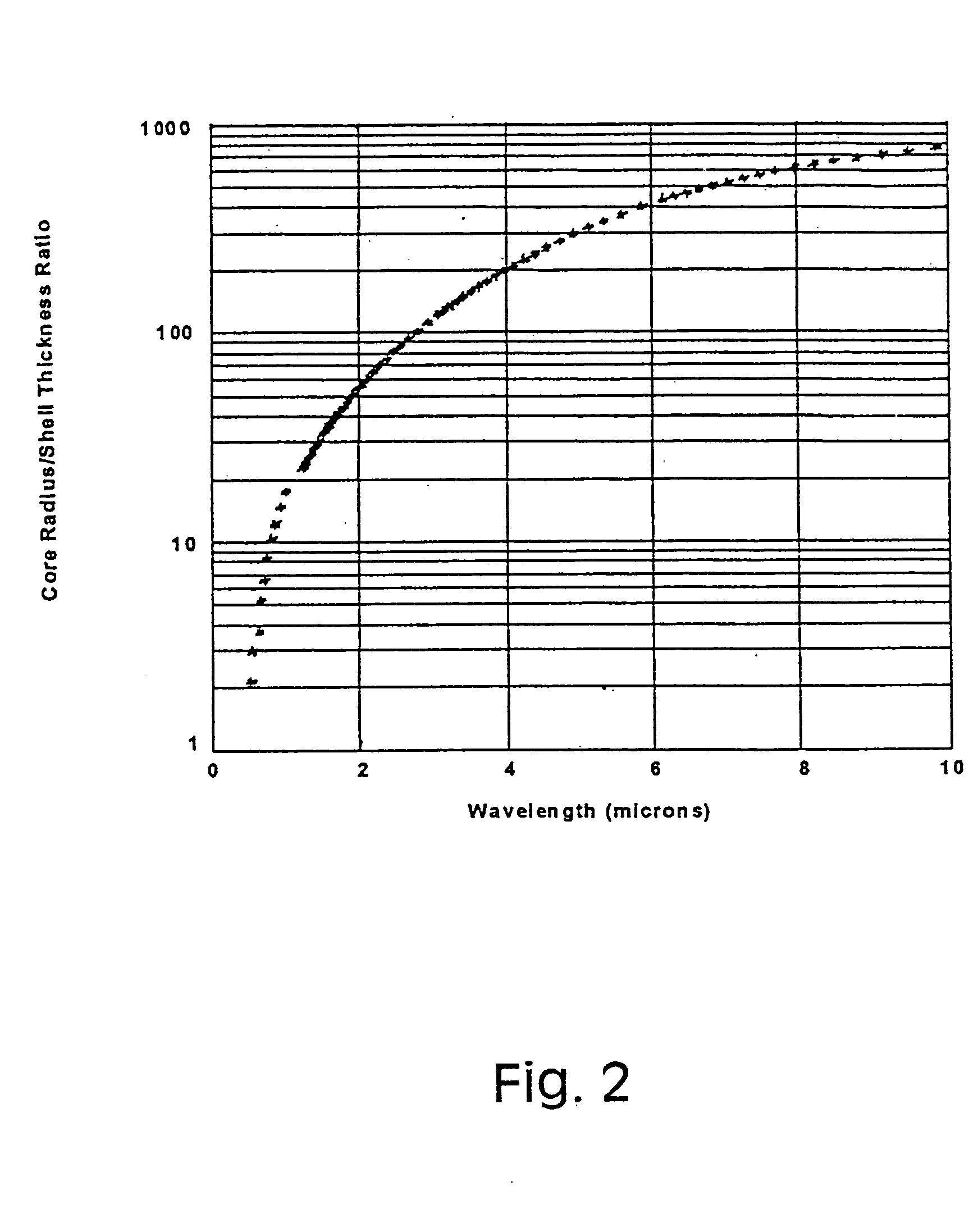
[0042] Since metal nanoshells have a plasmon resonance that is designed into the particle by adjusting the particle core:shell ratio, their plasmon resonance can be shifted during growth of the shell to coincide with the excitation wavelengths of near infrared laser sources, such as the 1064 nm Nd:YAG source used in a FT-Raman laser spectrometer.
[0043] In a series of recent experiments, the SERS enhancement properties of metal nanoshells were investigated (Oldenburg, S. J. et al. J. Chem. Phys. 111:4729-4735 (1999), incorporated in its entirety herein by reference). The nanoshell plasmon resonance was placed at nominally 900 nm, so that the shoulder of the plasmon peak overlapped with the Raman excitation wavelength. FIG. 5 shows the SERS enhancement observed in this study for the molecule mercaptoaniline. An enhancement of 600,000 in the Raman signal was observed. In this case, the strong interaction between mercaptoa...
example 2
Bioconjugation of Gold Nanoparticles / Nanoshells
[0044] Because the reduction of the outer metal layer of gold nanoshells is accomplished using the same chemical reaction as gold colloid synthesis, the surfaces of gold nanoshells are likely to be virtually chemically identical to the surfaces of the gold nanoparticles universally employed in conventional bioconjugate applications. Existing conjugation protocols for the labeling of a broad range of biomolecules with gold colloid (e.g., protein A, avidin, streptavidin, glucose oxidase, horseradish peroxidase and IgG) (M. A. Kerr et al., eds. Immunochemistry Labfax BIOS Scientific Publishers, Ltd., Oxford, U.K. 1994) will be directly repeatable or easily adaptable for use with gold nanoshells. Similar conjugation techniques are also expected to be readily adaptable for conjugation of nanoshells comprising other core materials. In one set of experiments, attachment of glucose oxidase (GO) to 150 nm diameter gold nanoshells was accomplish...
example 3
Gold Nanoshell-Based Biosensors
[0045] A preferred biosensing strategy combines the enormous SERS enhancements provided by metal nanoshells with the facile bioconjugation capabilities of gold nanoshell surfaces. This combination provides a highly sensitive, high information density spectral probe suitable for monitoring specific biochemical processes of physiological importance. Visible light is not suitable for in vivo optical monitoring due to its absorption by hemoglobin. Ultraviolet light is also not suitable due to the potential for photochemical transformation of proteins and DNA. Raman scattering in the near infrared, enhanced by the gold nanoshell plasmon resonance, lacks these disadvantages and is predicted by the inventors to facilitate the same demonstrated SERS sensitivity in regions of high physiological transmissivity, such as the “water windows” of 800-1,300 nm and 1,600-1,850 nm (Anderson, R. R. et al. J. Invest. Dermatol. 77:13-19 (1981); and Duck. F. A., Physical P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com