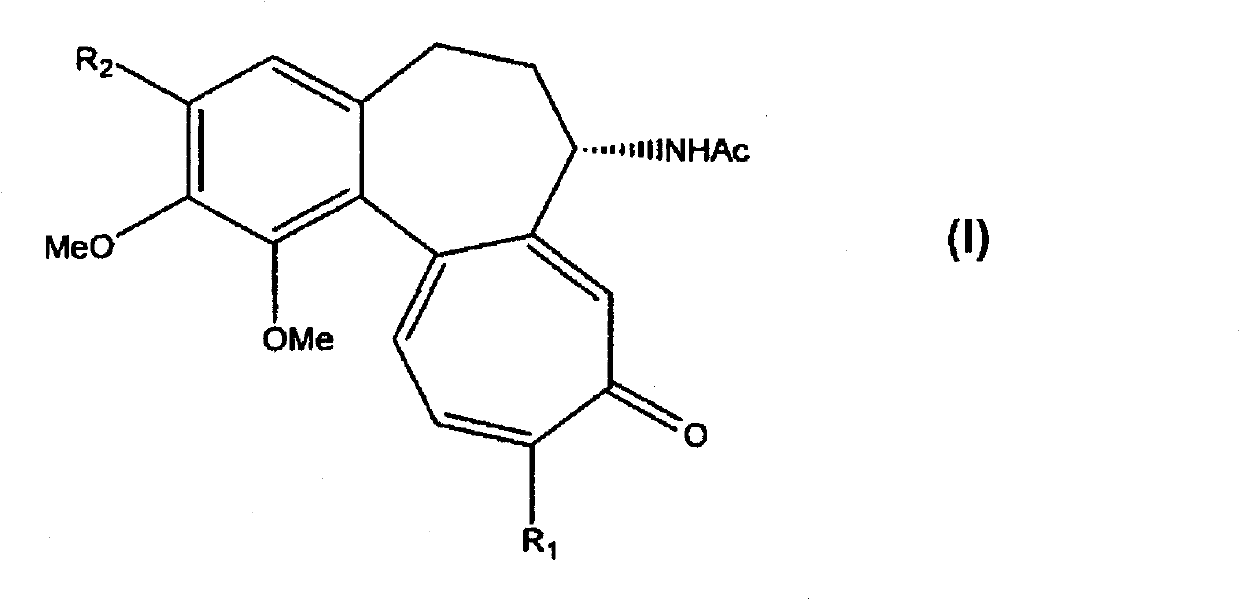
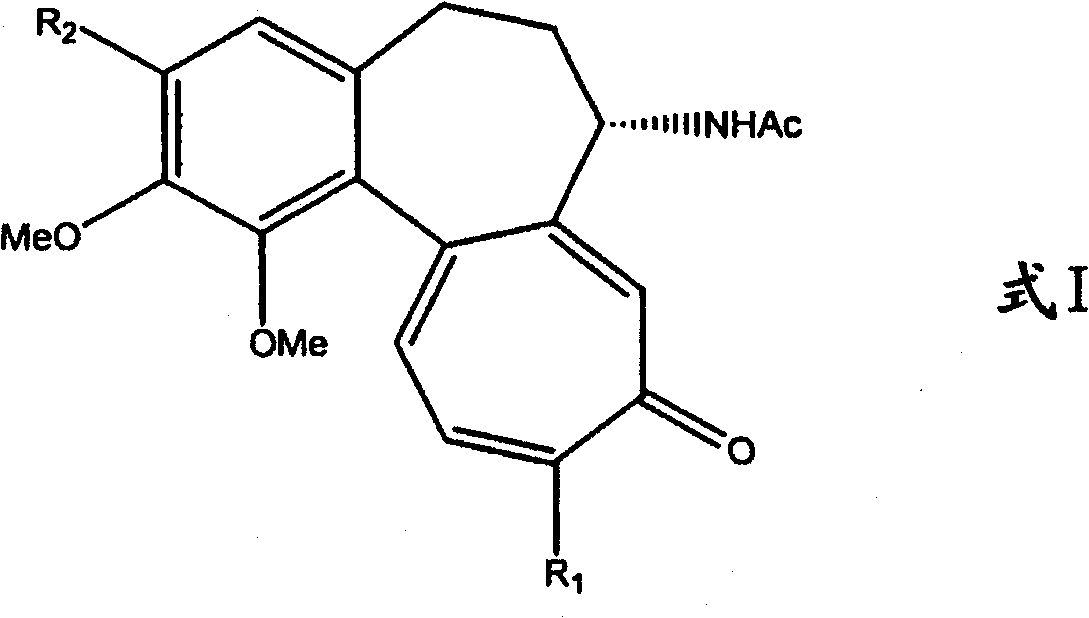
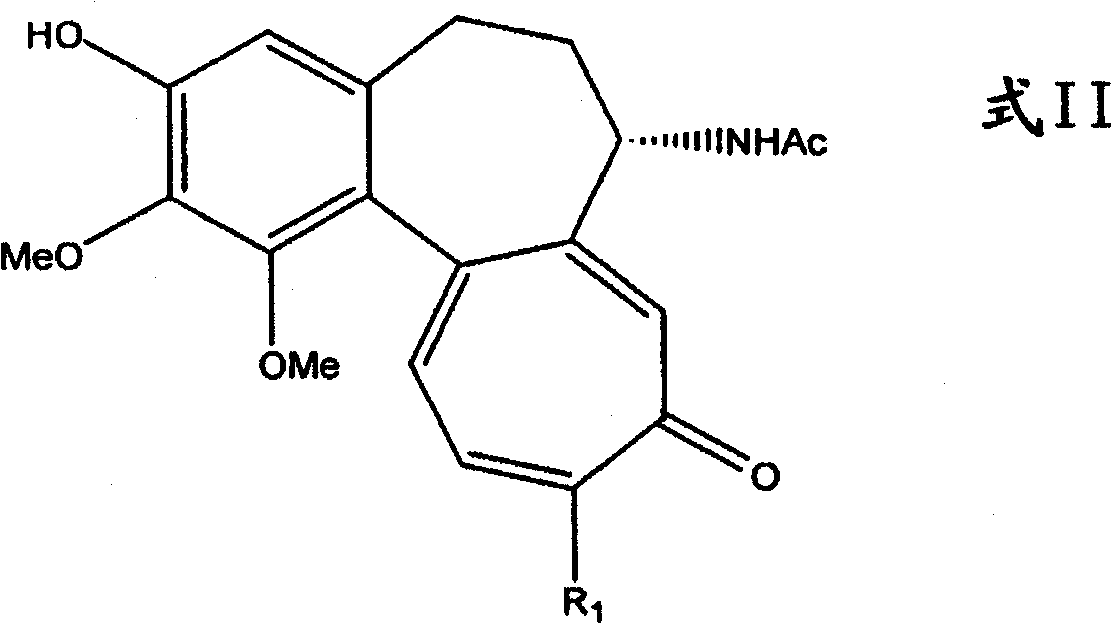
Process for the glycosidation of colchicine and thiocolchicine
一种化合物、甲硫基的技术,应用在制备秋水仙碱和硫秋水仙碱糖苷及其衍生物领域,能够解决冗长时间、产率低、不令人满意等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0027] The following examples illustrate the invention in more detail.
[0028] 1) Synthesis of thiocolchicoside (3-O-β-D-glucopyranosyl-3-O-demethylthiocolchicine)
[0029] Suspend 3-O-demethylthiocolchicine (2.0 g) in acetonitrile (20 ml) at room temperature under a nitrogen atmosphere, and then add 1,1,3,3-tetramethylguanidine (1.8 ml) in sequence A solution of 1,2,3,4,6-penta-O-acetyl-β-D-glucopyranose (5.60 g) in acetonitrile (10 ml) and finally boron trifluoride (7.2 ml).
[0030] The reaction mixture was stirred at room temperature for 2 hours, then cooled to 5°C and quenched by adding 2M KOH to pH ≈ 6 (approximately 20 ml). The aqueous layer was separated and extracted with acetonitrile (10ml). The combined organic layers were sequentially washed with NaHSO 4 0.5M (20ml), NaHCO 3 6% (20ml) and brine (20ml) washes.
[0031] The solvent was removed under vacuum and replaced with 95% ethanol (30ml). 2M NaOH (40ml) was added and the solution was stirred until compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com