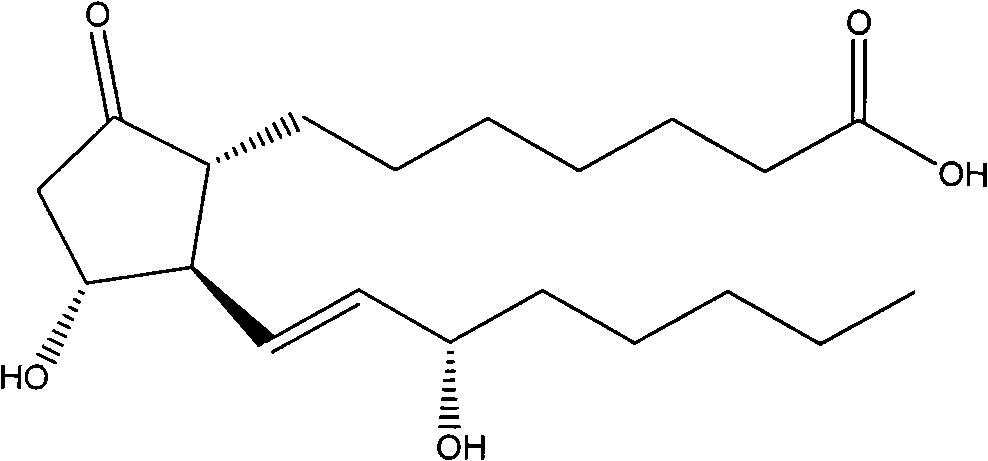
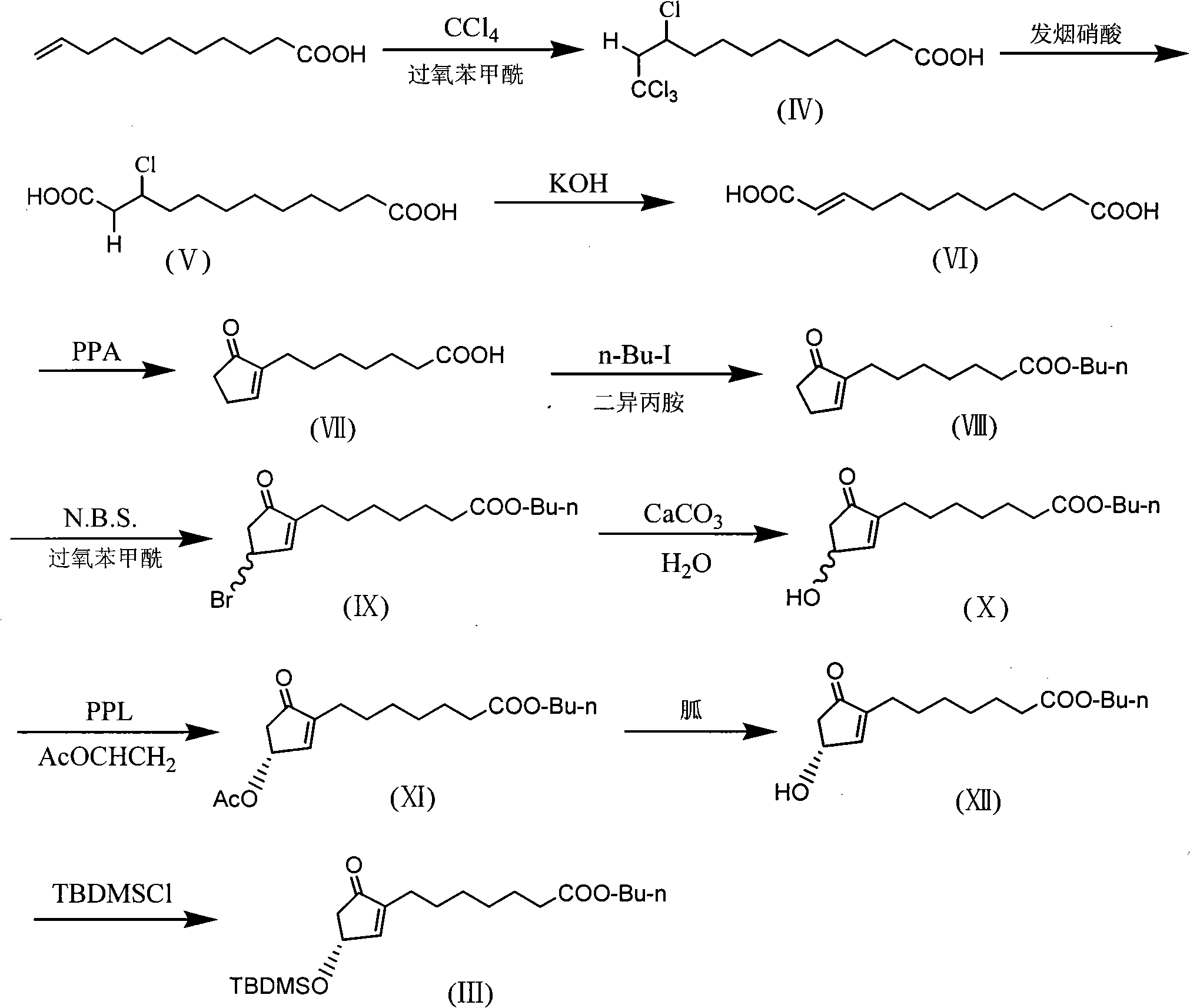
Method for preparing prostaglandin derivative
The technology of a compound and structural formula is applied in the field of synthesis of prostaglandin derivatives, which can solve the problems of small aortic effect and lower blood pressure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] 1. Preparation of 10,12,12,12-tetrachlorododecanoic acid (IV):
[0029] Put 400g of undecylenic acid, 640ml of dry carbon tetrachloride and 20g of benzoyl peroxide into a 2000ml round bottom flask, and heat to reflux for 15 hours. After the reaction was completed, it was naturally cooled to room temperature and stirred for 6 hours. The remaining carbon tetrachloride was recovered, and the carbon tetrachloride was removed by rotary evaporation under reduced pressure below 30°C to obtain 690 g of a yellow oily product. The yield was 94.0%. It can be directly used in the next reaction without further purification.
[0030] 2, the preparation of 3-chlorododecanedioic acid (V):
[0031] Put 690g of 10,12,12,12-tetrachlorododecanoic acid into a 2000ml three-neck bottle, add 1017ml of fuming nitric acid dropwise at room temperature, and control the temperature at 25-30°C. There is a little yellow gas shrouded on it, and the dripping is completed in about 6 hours. At room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com