Application of 4-alkyl-5-(1,2,4-triazole-1-yl)-2-benzyl imino thiazole in preparation of weedicide
A technology of benziminothiazole and herbicide, applied in the field of application of 4-alkyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole as the preparation of herbicide, Can solve the problem of no research and development reports on herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
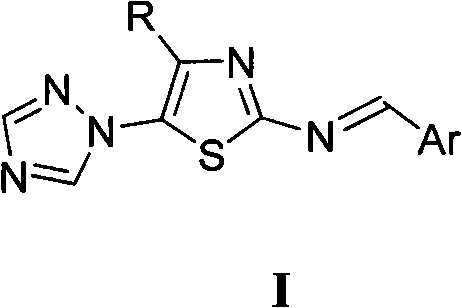
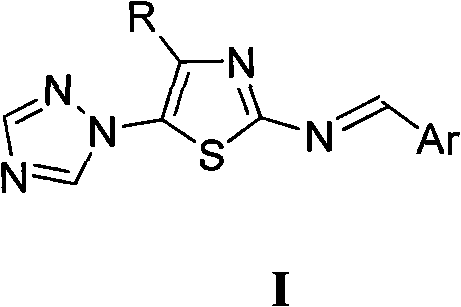
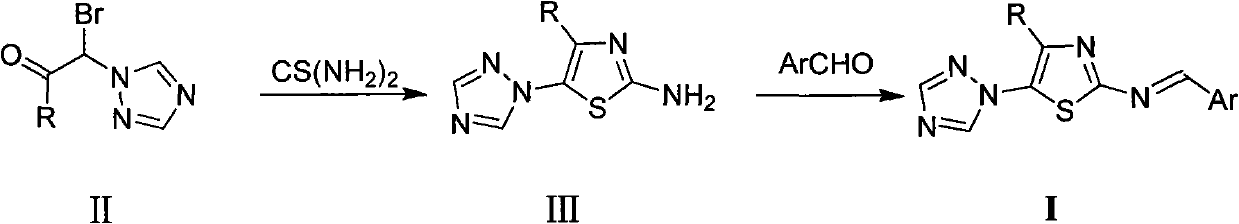
[0010] Preparation of Example 14-Alkyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole (I)
[0011] The preparation method of 4-alkyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole (I) is carried out according to the following reaction formula:
[0012]
[0013] In the reaction formula, R is selected from: C 1~2 Alkyl, C 3~4 Straight chain or branched chain alkyl; Ar is selected from: phenyl, 2-chlorophenyl, 2-fluorophenyl, 2-hydroxyphenyl, 2-methoxyphenyl, 2-ethoxyphenyl, 2 -nitrophenyl, 3-dimethylaminophenyl, 3-chlorophenyl, 3-bromophenyl, 3-fluorophenyl, 3-methylphenyl, 3-ethylphenyl, 3-trifluoro Methylphenyl, 3-hydroxyphenyl, 3-methoxyphenyl, 3-ethoxyphenyl, 3-nitrophenyl, 3-sulfonylphenyl, 3-methanesulfonylaminophenyl , 3-sulfamoylphenyl, 4-dimethylaminophenyl, 4-chlorophenyl, 4-bromophenyl, 4-fluorophenyl, 4-methylphenyl, 4-ethylphenyl, 4 -Trifluoromethylphenyl, 4-hydroxyphenyl, 4-methoxyphenyl, 4-ethoxyphenyl, 4-acetoxyphenyl, 4-nitrophenyl, 4-sulfonic acid Phenyl, 4-met...
Embodiment 24
[0015] Determination of herbicidal activity of embodiment 24-alkyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole
[0016] 1 Purpose of the test
[0017] The toxicity of new compounds to Abutilon, Amaranth, Chenopodium, Digitata and Dogtail was screened indoors, and their herbicidal activity was evaluated.
[0018] 2 test conditions
[0019] 2.1 Test target
[0020] Targets are Abutilon theophrasti, Amaranthus spinosus L, Chenopodium album, Digitaria sanguinalis and Setaria viridis;
[0021] 2.2 Culture conditions
[0022] The culture conditions of the tested target and the target after the test are a temperature of 20±5° C. and a relative humidity of 65±5%.
[0023] 2.3 Instruments and equipment
[0024] Electronic balance (sensitivity of 1 / 10,000), 100ml beaker, measuring cylinder, crop sprayer, etc.
[0025] 3 Experimental design
[0026] 3.1 Test drug 4-alkyl-5-(1,2,4-triazol-1-yl)-2-benziminothiazole.
[0027] 3.2 Test concentration A single dose of 2250g ai / ha was se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com