Resolution method for preparing optically pure metoprolol
A pure and optical technology, applied in the field of splitting racemic metoprolol to prepare its S- and -R--metoprolol isomers, can solve the problem of low optical purity and achieve small loss of resolving agent , high splitting efficiency, and the effect of saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
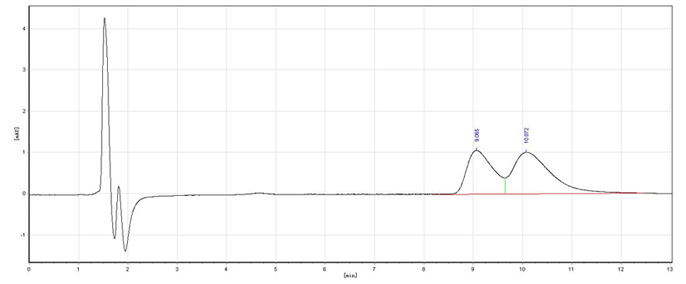
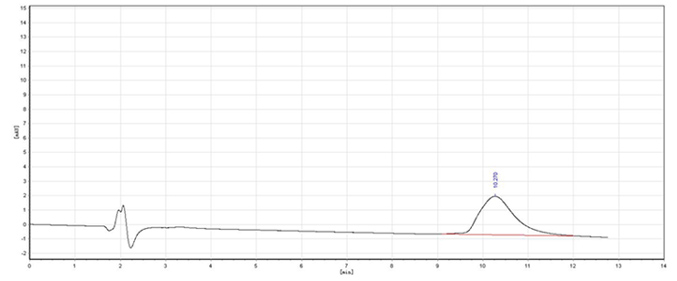
Image
Examples
Embodiment 1
[0023] (1) Add 267.37g (1mol) of racemic metoprolol and 242.35g (1mol) of (-)-4-phenyl-2-hydroxy-5,5-dimethyl in 2L of methyl tert-butyl ether base-2-oxo-1,3,2-dioxaphosphorinane, heated to reflux and stirred until the solution was clear, continued to stir for 1h, crystals gradually precipitated, filtered, and washed the filter cake with 200mL methyl tert-butyl ether , to obtain filter cake 244.5g after drying;
[0024] (2) Dissolve the above filter cake into 500mL water, adjust the pH=8 with 10% NaOH solution, stir at room temperature for 1h, extract three times with ethyl acetate, each time 100mL, dry the organic layer, and evaporate the organic solvent to obtain S -(-)-Metoprolol 120.33g, the yield is 90.2%, 96% e.e. . The pH of the water layer is adjusted to strong acidity, and solids are gradually precipitated, filtered, and the cyclic phosphoric acid resolving agent is recovered;
[0025] (3) Combine the filtrate and washing liquid in step (1), evaporate the organic ...
Embodiment 2
[0028] (1) Add 267.37g (1mol) racemic metoprolol and 276.5g (1mol) (+)-4-(2-chlorophenyl) in 2L ether / ethanol solution (volume ratio 9:1) -2-Hydroxy-5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinane, heated to reflux and stirred until the solution was clear, continued to stir for 1h, crystals gradually precipitated, filtered, The filter cake was washed with 200 mL of ether, and 250.5 g of the filter cake was obtained after drying;
[0029] (2) Dissolve the above filter cake in 500mL of water, adjust the pH=8 with 10% KOH solution, stir at room temperature for 1h, extract three times with ethyl acetate, 100mL each time, dry the organic layer, and evaporate the organic solvent to obtain R-(+)-metoprolol 122.33g, the yield is 91.5%, 96.4% e.e. . The pH of the water layer is adjusted to strong acidity, and solids are gradually precipitated, filtered, and the cyclic phosphoric acid resolving agent is recovered;
[0030] (3) Combine the filtrate and lotion in step (1), distill off the...
Embodiment 3
[0033] (1) Add 267.37g (1mol) of racemic metoprolol and 275.4g (1mol) of combined cyclic phosphoric acid resolving agent into 2L ether / methanol solution (volume ratio: 9:1), heat and reflux and stir until the solution is clear , continue to stir for 1h, crystals gradually precipitate out, filter, wash the filter cake with 200mL ether, and obtain 257.82g of the filter cake after drying;
[0034] The combined cyclic phosphoric acid resolving agent is 98% (-)-4-(2-chlorophenyl)-2-hydroxyl-5,5-dimethyl-2-oxo-1,3,2-di Oxaphosphorinane and 2% of (-)-4-(2-methoxyphenyl)-2-hydroxy-5,5-dimethyl-2-oxo-1,3,2- Dioxaphosphine, the molar ratio of the two is 98: 2;
[0035] (2) Dissolve the above filter cake in 500mL of water, add 10% ammonia solution to adjust pH = 8, stir at room temperature for 1h, extract three times with ethyl acetate, 100mL each time, dry the organic layer, evaporate the organic solvent to obtain S- (-)-metoprolol 126.33g, the pH of the water layer was adjusted to st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com