Method for simultaneously preparing chemical reference substances of calycosin and formononetin
A technology of calycosin and formononetin, applied in the direction of organic chemistry, can solve the problems of cumbersome steps and low purity of reference substances, and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Silica gel column chromatography enrichment:
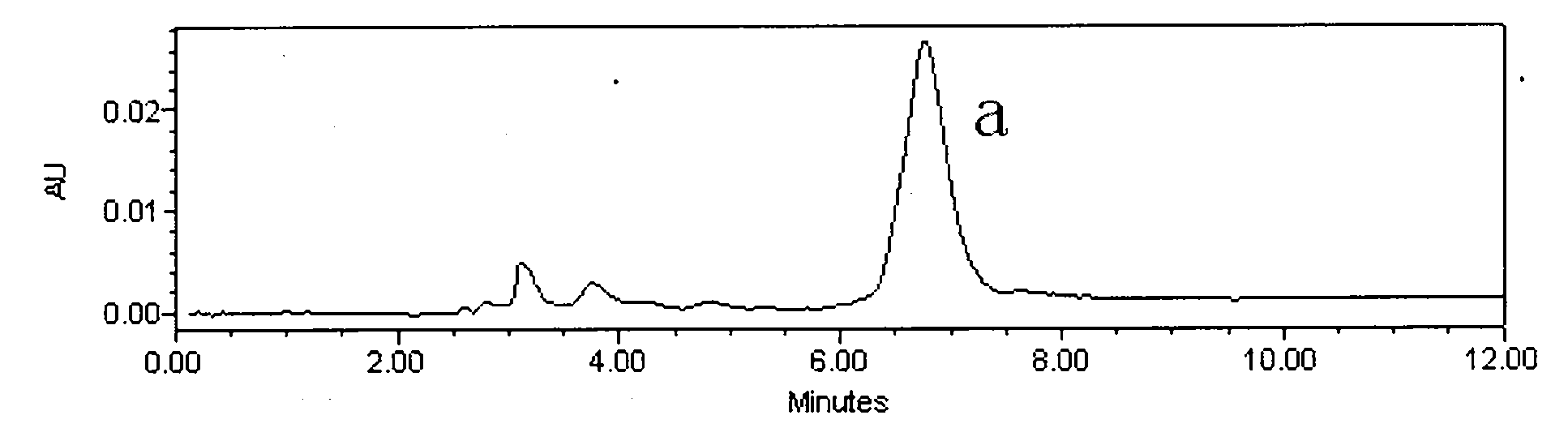
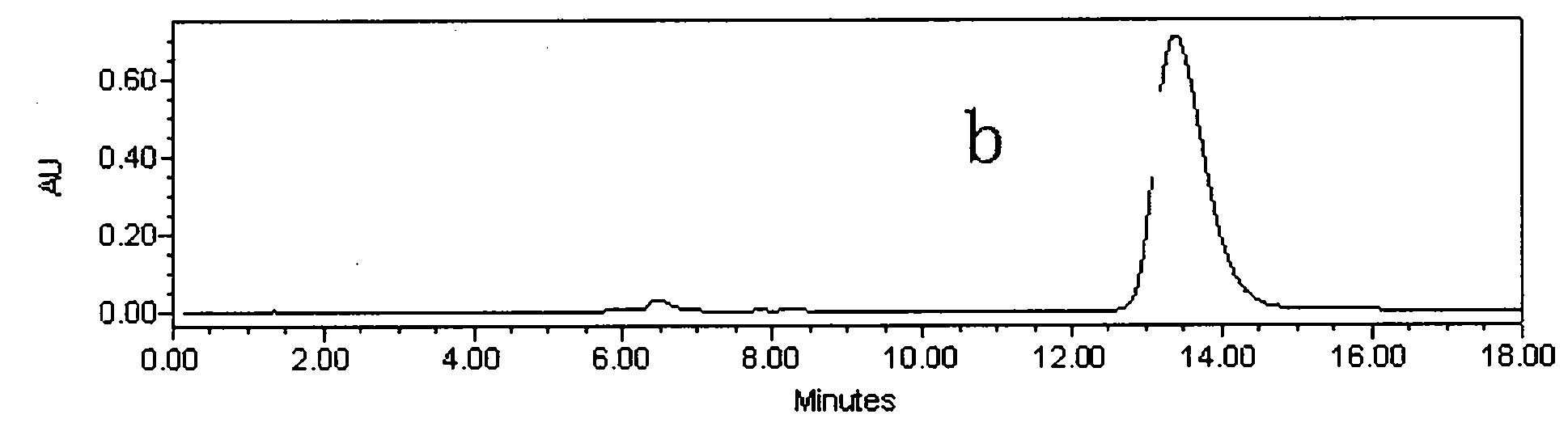
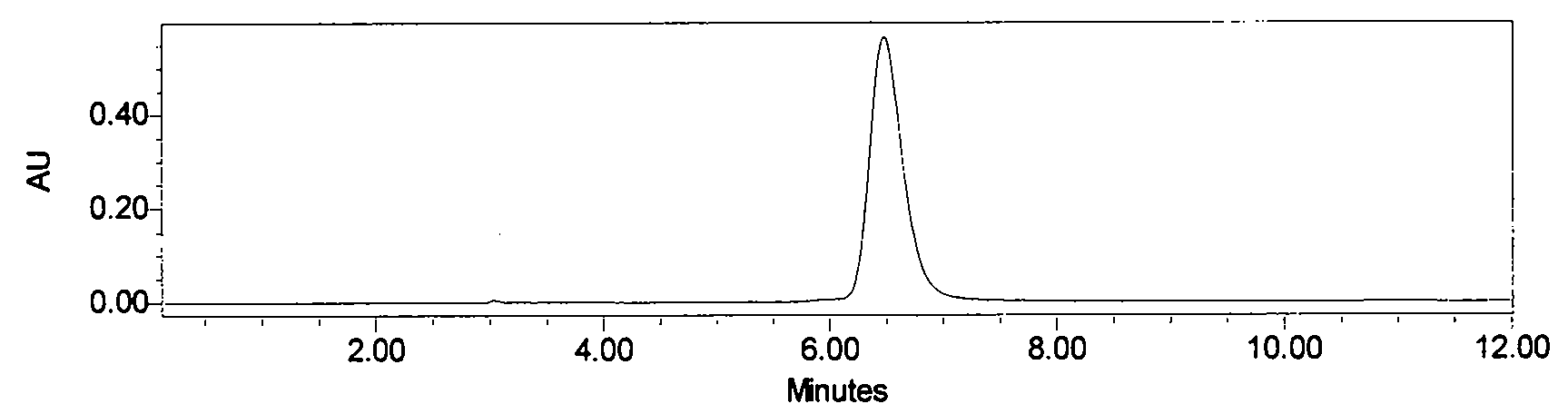
[0024] Astragalus alcohol extract extract 70g, add about 70g silica gel and mix well, dry under reduced pressure at 60°C, separate by silica gel vacuum chromatography (21cm*12cm), use 5 times of column volumes of petroleum ether: ethyl acetate (volume ratio 8 : 2), petroleum ether: ethyl acetate (volume ratio 7: 3), petroleum ether: ethyl acetate (volume ratio 5: 5) step gradient elution, thin-layer chromatography detection, with petroleum ether: ethyl acetate (volume Ratio 1:1) as developer, GF254 fluorescent plate, dark spots were inspected at 254nm of ultraviolet light, the eluate with Rf value of 0.13 was collected, concentrated, and 35mg of light yellow precipitate was separated out (the crude product of calycosin, figure 1 ); collect the eluent whose Rf value is 0.44, concentrate, and separate out light yellow precipitate 11mg (the crude product of formononetin, figure 2 );
[0025] 2) Recrystallization:
[002...
Embodiment 2
[0029] 1) Silica gel column chromatography enrichment:
[0030] Astragalus ethanol extract extract 700g, add about 700g silica gel and mix well, dry under reduced pressure at 60°C, separate by silica gel column chromatography (8cm*80cm), use 4 times the column volume of petroleum ether: ethyl acetate (volume ratio 8: 2), petroleum ether: ethyl acetate (volume ratio 7: 3), petroleum ether: ethyl acetate (volume ratio 5: 5) step gradient elution, thin-layer chromatographic detection, with petroleum ether: ethyl acetate (volume ratio 3:7) as developer, GF254 fluorescent plate, dark spots were inspected at 254nm of ultraviolet light, the eluate with Rf value of 0.32 was collected, concentrated, and 360 mg of pale yellow precipitate (crude product of calycosin) was separated out; the eluate with Rf value of 0.64 was collected Deliquified, concentrated, and 105 mg of light yellow precipitate (crude formononetin) was precipitated;
[0031] 2) Recrystallization:
[0032] The crude p...
Embodiment 3
[0034] 1) Silica gel column chromatography enrichment:
[0035] Astragalus ethanol extract extract 7000g, add about 7000g silica gel and mix well, dry under reduced pressure at 60°C, separate by silica gel column chromatography (9.5cm*120cm), use 3 times the column volume of petroleum ether: ethyl acetate (volume ratio 8 : 2), petroleum ether: ethyl acetate (volume ratio 7: 3), petroleum ether: ethyl acetate (volume ratio 5: 5) step gradient elution, thin-layer chromatography detection, with petroleum ether: ethyl acetate (volume Ratio 1:1) as developing agent, GF254 fluorescent plate, dark spots were inspected at 254nm of ultraviolet light, the eluate with Rf value of 0.20 was collected, concentrated, and 3.58g of light yellow precipitate (crude product of calycosin) was precipitated; the collected Rf value was 0.48 The eluent was concentrated, and 1.1 g of a light yellow precipitate (crude formononetin) was separated out;
[0036] 2) Recrystallization:
[0037] The crude p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com