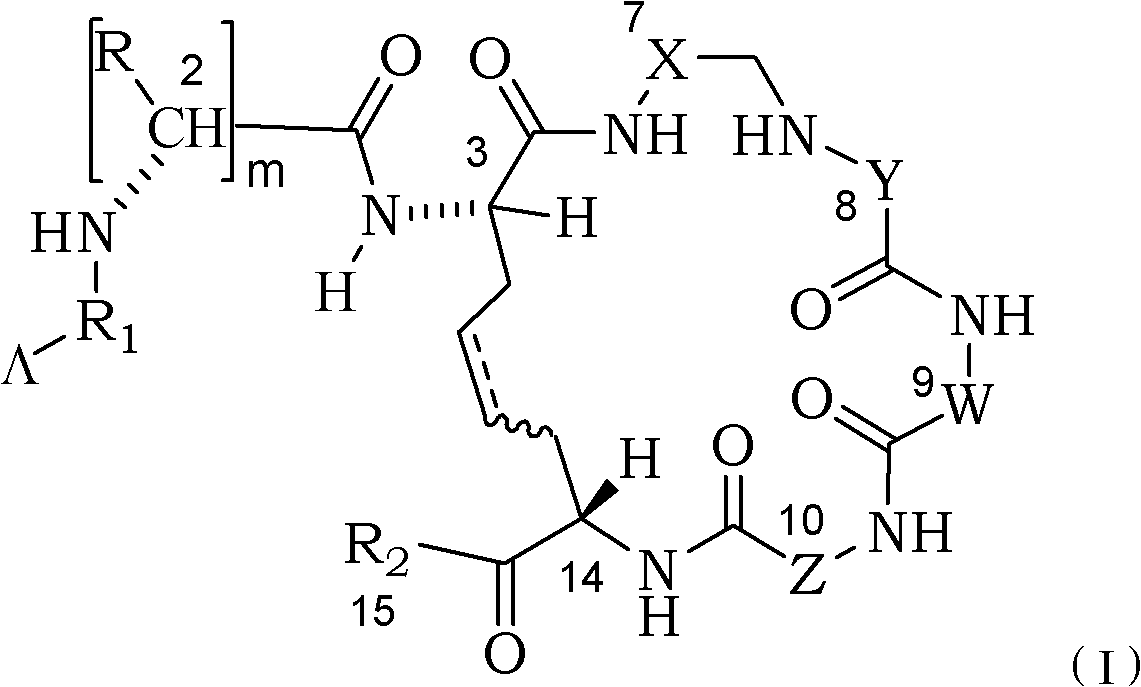
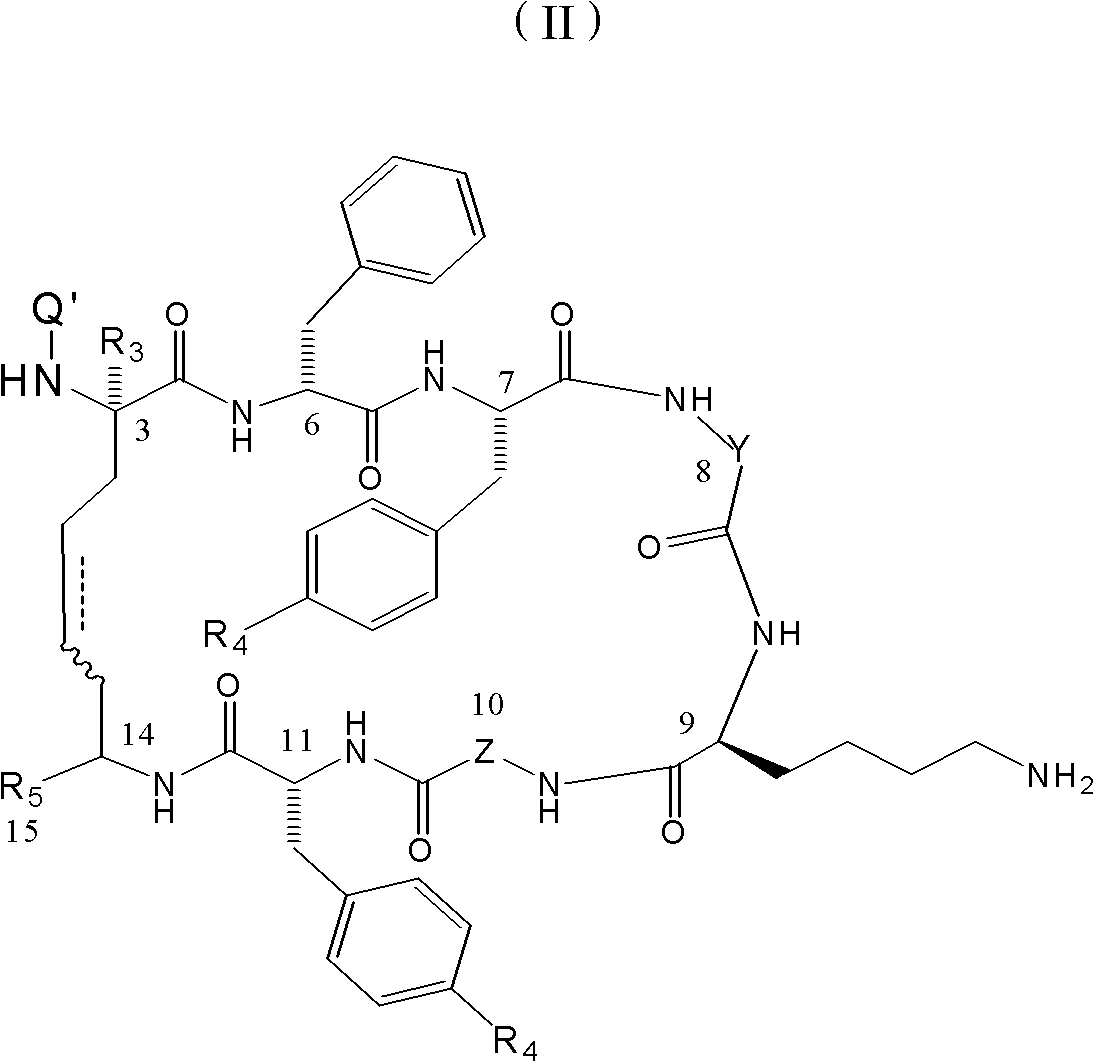
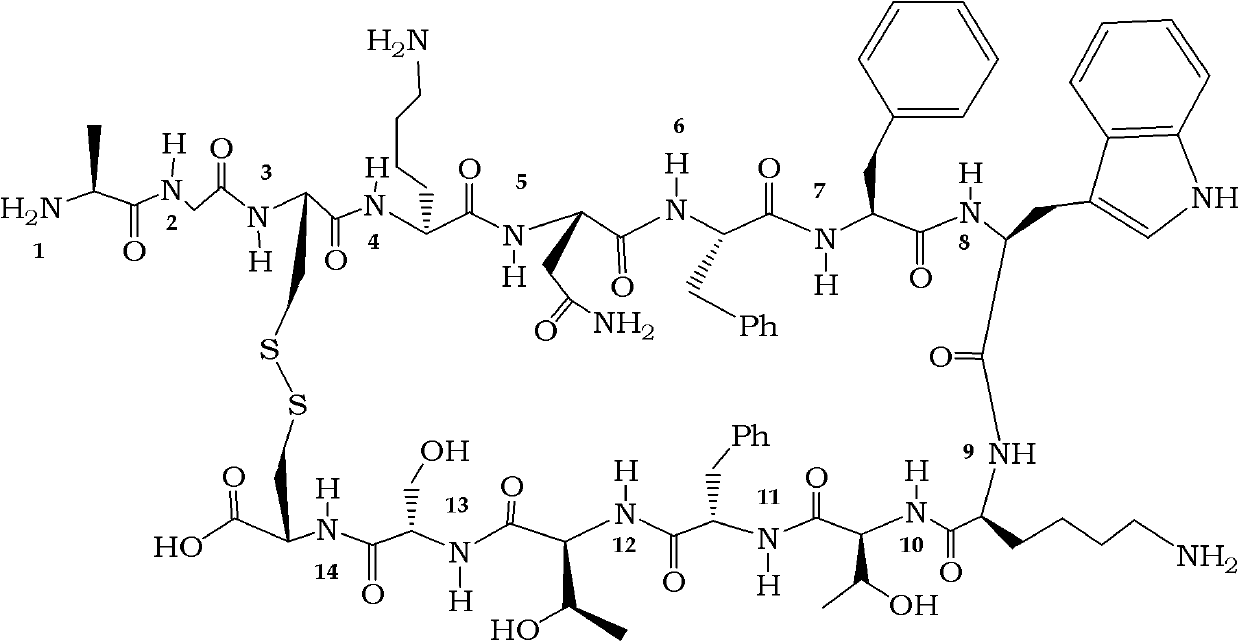
Dicabba-analogues of octreotide
A compound and chiral carbon technology, applied in the field of analog derivatives of octreotide, can solve problems such as short half-life and clinical use restrictions of somatostatin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0076] Linear heptapeptide Fmoc-Hag 3 (Allyl amino acid)-1-Nal 7 -D-Trp 8 -Lys 9 -Thr 10 -Hag 14 -Thr(ol) 15 Synthesis
[0077] Peptides were prepared in Teflon reactors with porous polystyrene membranes using the Fmoc-SPPS method on pre-swelled H-L-Thr(tBu)-ol-2-chlorotriphenyl resin.
[0078] Coupling steps were performed by adding 2 equivalents of protected amino acid, activated by HATU and 4 equivalents of NMMDMF solution, followed by stirring for 45 minutes for each coupling and monitored by qualitative tests using ninhydrin (Kaiser test).
[0079] Once the synthesis of the linear peptide was complete, microlysis was performed on 5 mg of resin. This resin uses TFA / H 2 Treated with O / 1,2-ethanedithiol (EDT) / phenol (94:2:2:2; 3h) and filtered, the solution was concentrated under reduced pressure and washed with Et 2O precipitated peptides, centrifuged, dissolved in water and lyophilized. Analysis of Fmoc-protected crude peptides by RP-HPLC [Phenomenex Jupiter C18 ...
example 2
[0082] Synthesis of cyclic unsaturated peptides of structural formula (1), wherein -Λ is H, R=benzyl, R 1 is a chemical bond, CH 2 is the D configuration, CH 3 It is L configuration, X=1-Nal, Y=D-Trp, W=Lys, Z=Thr, R 2 =-NHThr(ol)
[0083] The heptapeptide on resin was re-swelled in anhydrous DCM for 2 hours. The reactor was heated to 45 °C and a solution of the second generation Grubbs catalyst, [(L)(L')X 2 Ru=CHR wherein L is a phosphine ligand, L' is a heterocyclic carbene (1,3-bis(2,4,6-trimethylphenyl)-imidazole-2-ylidene) (1,3-dimesityl-imidazol -2-ylidene), X is a Cl atom and R is a phenyl] (0.5 molar equivalent), and the suspension was stirred for 24 hours. The resin was washed with DCM, DMF and MeOH, followed by swelling with DMF. Fmoc-Hag was deprotected with 20% piperidine, and the cyclic peptide coupled to Fmoc-D-Phe was as described previously. This cyclic peptide was deprotected and isolated from the resin as previously described. with Et 2 O concentrate...
example 3
[0086] Synthesis of the unsaturated cyclic peptide of structural formula (1), wherein-Λ is a chelating group DOTA, R=benzyl, R 1 is a chemical bond, CH 2 is the D configuration, CH 3 It is L configuration, X=1-Nal, Y=D-Trp, W=Lys, Z=Thr, R 2 =-NHThr(ol)
[0087] As described in Example 2, the Fmoc-D-Phe of formula I bonded to the resin 2 The deprotection of the peptide was carried out with 20% piperidine; then 2 molar equivalents of DOTA-tri-(t-Bu) ester in DMF, 2 molar equivalents of HATU in DMF and then 4 molar equivalents of NMM were added to in the resin. The equivalent is calculated based on a resin substitution rate of 0.5 mmol / g. The DOTA-conjugated peptide was separated from the resin after the resin was washed for 45 minutes. The tri-(t-Bu)DOTA-protecting group was isolated together with the peptide. The crude peptide was dissolved in water and lyophilized, then purified by RP-HPLC (20%-60% of B in 20 minutes; yield: 29%).
[0088] ESI-MS: found [M+H] + 1417....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com