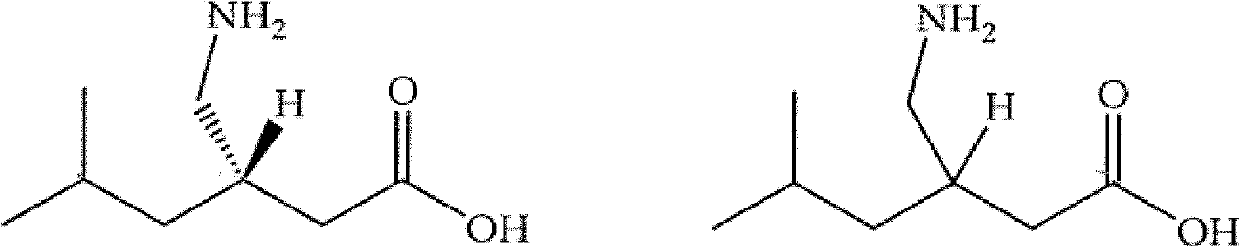
Novel process
An enantiomeric, enriched technology, applied in the field of γ-amino acids such as enantiomerically enriched-pregabalin, which can solve problems such as expensive resolving agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
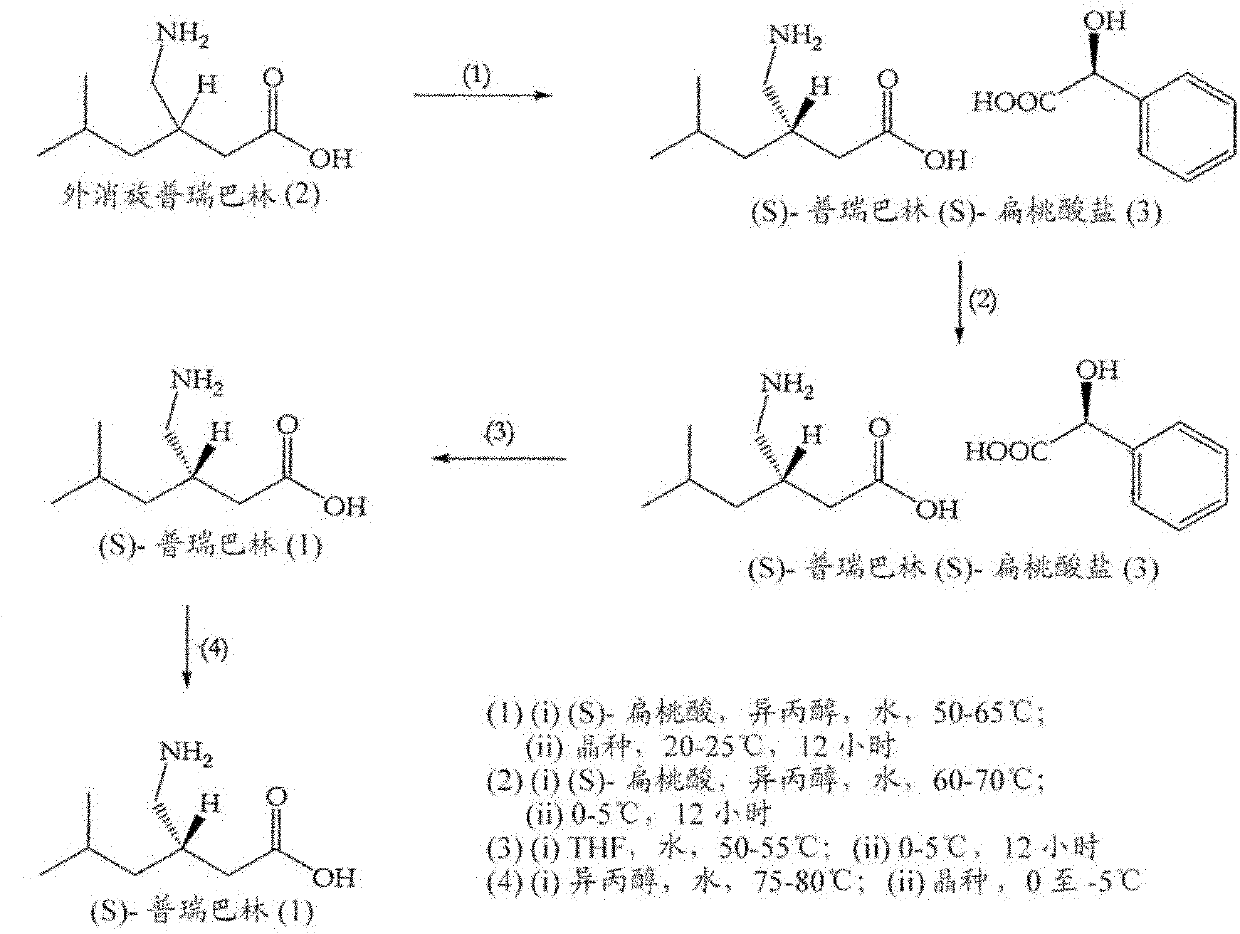
[0168] Example 1: Resolution of racemic pregabalin using L-tartaric acid by the method described in Scheme 2 (2)
[0169] Preparation of (S)-pregabalin (L)-tartrate (4):
[0170] A mixture of racemic 3-aminoethyl-5-methylhexanoic acid (2) (100 g), L-tartaric acid (94.3 g), n-butanol (1.01) and water (200 ml) was stirred at 25-30°C to obtain a clear solution. The clear solution was then filtered, cooled to 8-10°C, and stirred at 8-10°C for 4 hours. The solid obtained was filtered off and dried under vacuum at 40-45°C. Yield: 90 g (93% mol, 46% w / w). Enantiomeric purity: 99.0% S-isomer (as determined by chiral HPLC). Chemical purity: 99.64% (as determined by HPLC). No lactam impurity was observed by HPLC.
[0171] Recrystallization of (S)-pregabalin (L)-tartrate (4):
[0172] (S)-Pregabalin (L)-tartrate (4) (90 g) was added to n-butanol (450 ml) and water (90 ml) and stirred at 20-30° C. to obtain a clear solution. The clear solution was filtered, cooled to 10-15°C, a...
Embodiment 2
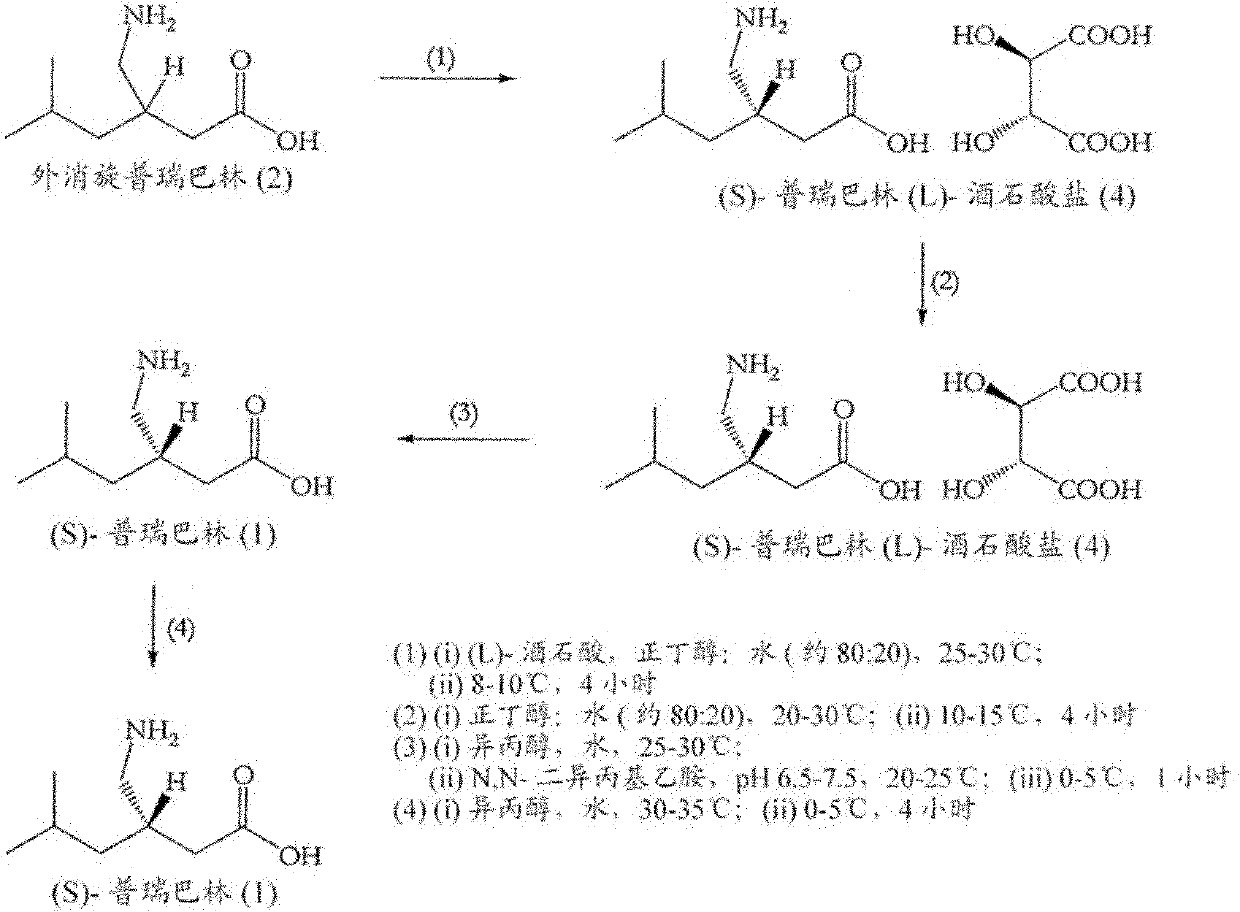
[0177] Example 2: Resolution of racemic pregabalin by L-tartaric acid by the method described in Scheme 3 (2)
[0178] Preparation of pregabalin (L)-tartrate (5):
[0179] A mixture of racemic 3-aminoethyl-5-methylhexanoic acid (2) (100 g), L-tartaric acid (94.3 g), n-butanol (1.01) and water (100 ml) was stirred at 20-25°C to obtain a clear solution. The clear solution was then filtered, cooled to 0-5°C, and stirred at 0-5°C for 4 hours. The solid obtained was filtered off and dried under vacuum at 40-45°C. Yield: 184.5 g (95% mole and w / w). Enantiomeric purity: 48% (S)-pregabalin (L)-tartrate and 52% (R)-pregabalin (L)-tartrate (as determined by chiral HPLC). Chemical purity: 99.91% (as determined by HPLC). No lactam impurity was observed by HPLC.
[0180] Preparation of (S)-pregabalin (L)-tartrate (4) by fractional crystallization:
[0181] Pregabalin (L)-tartrate (5) (184.5g) was added to n-butanol (1845ml) and water (369ml) and stirred at 20-30°C to obtain a cl...
Embodiment 3
[0186] Example 3: Resolution of racemic pregabalin (2) by a method similar to that described in Scheme 2 but using O,O'-di-p-toluoyl-(D)-tartaric acid
[0187] (S)-Pregabalin O, the preparation of O'-di-p-toluoyl-(D)-tartrate (6):
[0188] Stir racemic 3-aminoethyl-5-methylhexanoic acid (2) (100 g), O, O'-di-p-toluoyl-(D)-tartaric acid (126 g), tert-butyl A mixture of alcohol (1.01) and water (200ml) to obtain a clear solution. The clear solution was then filtered, cooled to 0-5°C, and stirred at 0-5°C for 4 hours. The solid obtained was filtered off and dried under vacuum at 40-45°C. Yield: 168 g (98% mole, 49% w / w). Enantiomeric purity: 96.0% S-isomer (as determined by chiral HPLC). Chemical purity: 99.50% (as determined by HPLC). No lactam impurity was observed by HPLC.
[0189] Recrystallization of (S)-pregabalin O.O'-di-p-toluoyl-(D)-tartrate (6):
[0190] (S)-Pregabalin O, O'-di-p-toluoyl-(D)-tartrate (6) (168g) was added in tert-butanol (1680ml) and water (33...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com