Traditional Chinese medicament freeze-drying injection and preparation method thereof
A technology for freeze-dried injections and traditional Chinese medicines, which can be used in freeze-dried delivery, pharmaceutical formulations, drug combinations, etc., and can solve problems such as undisclosed freeze-dried injection preparation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
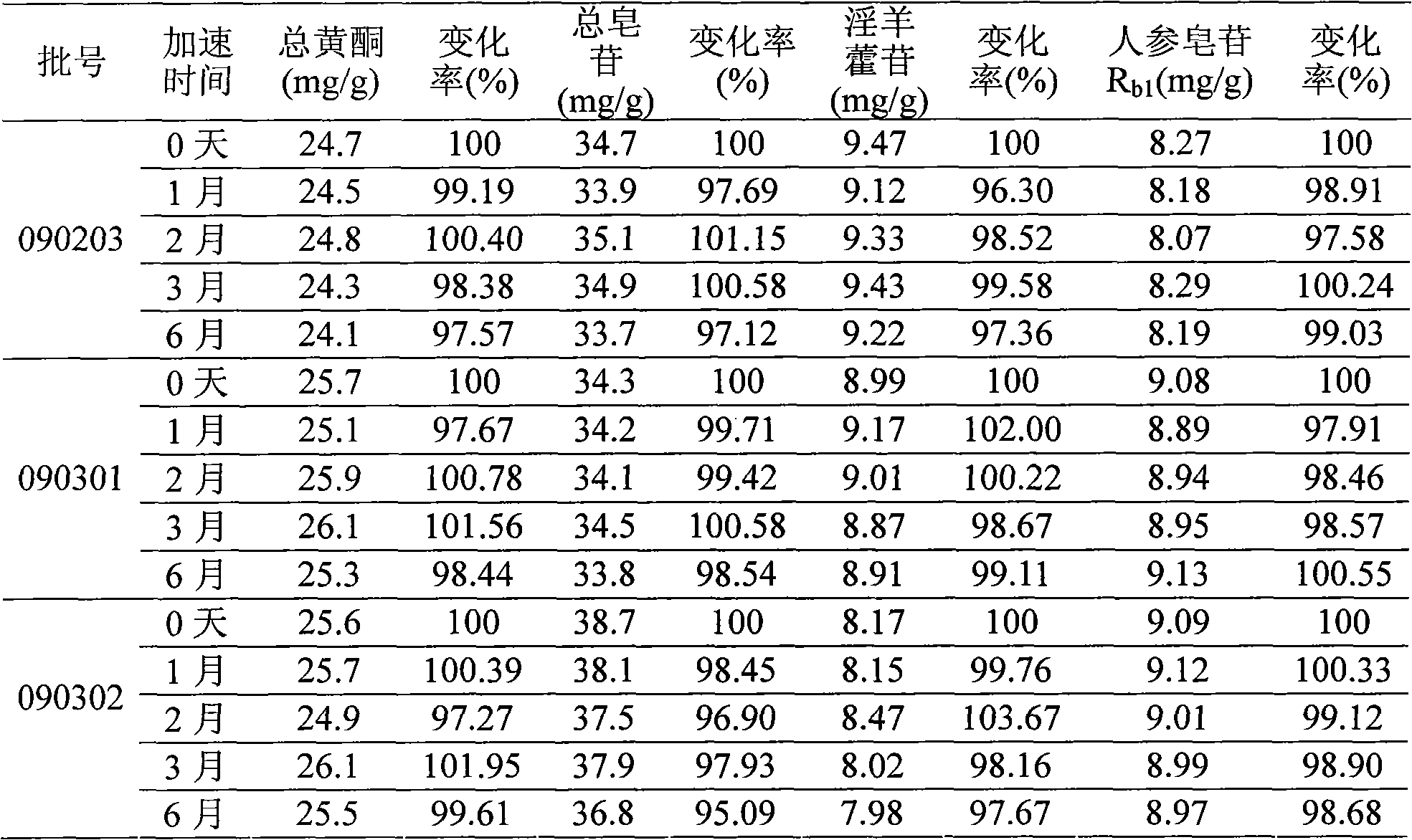
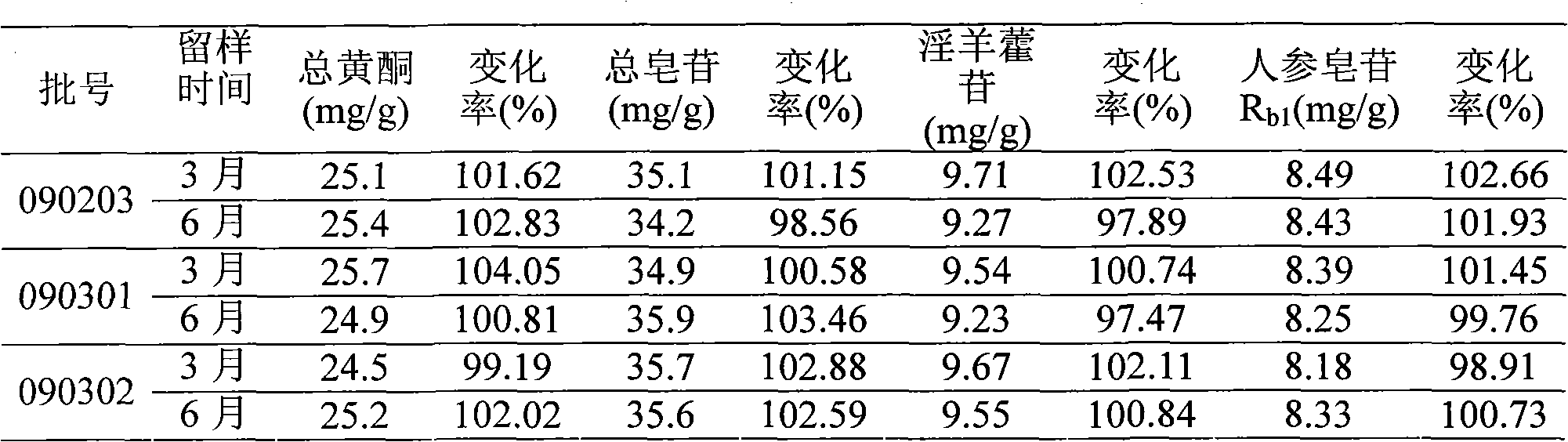
Image
Examples
Embodiment 1
[0058] Raw material weight ratio:
[0059] Ginseng total saponins extract 50g Epimedium total flavonoids extract 40g Hydroxypropyl-β-cyclodextrin 800g
[0060] Preparation:
[0061] a. Dissolve hydroxypropyl-β-cyclodextrin with 4000ml of water to make a 20% solution. Add 750ml (5.33%) of the total flavonoids extract of Epimedium to heat to dissolve, and slowly add hydroxypropyl-β-ring in portions In the dextrin solution, keep the temperature at 80°C±1°C, dynamically stir and clathrate for 3 hours, add 100ml (50%) of the total ginsenoside extract to water to dissolve it by heating, add it to the clathrate solution, and continue to clathrate for 1 hour;
[0062] b. Add water to adjust the total amount of the solution to 6000ml, adjust the pH to 7.5 with 10% sodium hydroxide solution, add 24 grams of activated carbon (that is, containing 0.4% activated carbon), boil for 10 minutes, filter with a clear plate while it is hot, and filter out the activated carbon , Then filter with 0.22μm m...
Embodiment 2
[0069] Raw material weight ratio:
[0070] Total ginseng saponins extract 35 grams Epimedium total flavonoids extract 55 grams Hydroxypropyl-β-cyclodextrin 900 grams
[0071] Preparation:
[0072] a. Dissolve hydroxypropyl-β-cyclodextrin in 3000ml of water to make a 30% solution, add 1800ml (3.11%) of the total flavonoids extract of Epimedium to dissolve in water, and slowly add hydroxypropyl-β-cyclodextrin in portions In the refined solution, keep the temperature at 50±1°C, dynamically stir the inclusion for 8 hours, add 105ml (33.3%) of the total ginsenoside extract and heat to dissolve it, add it to the inclusion solution, and continue the inclusion for 2 hours;
[0073] b. Add water to adjust the total amount of the solution to 6000ml, adjust the pH to 6.2 with 20% sodium hydroxide solution, add 6 grams of activated carbon (containing 0.1% activated carbon), boil for 10 minutes, filter with a sand filter while hot, and filter out the activated carbon. Then filter with 0.22μm micr...
Embodiment 3
[0080] Raw material weight ratio:
[0081] Ginseng total saponins extract 65g Epimedium total flavonoids extract 25g Hydroxypropyl-β-cyclodextrin 650g
[0082] Preparation:
[0083] a. Dissolve hydroxypropyl-β-cyclodextrin with water 4000 to make a 16.3% solution, add 250ml (10%) of total flavonoids extract of Epimedium to heat to dissolve, add hydroxypropyl-β-ring slowly in batches In the dextrin solution, keep the temperature at 90°C±1°C, dynamically agitate and clathrate for 3 hours. Add 130ml (50%) of total ginsenoside extract to heat to dissolve it, add it to the clathrate solution, and continue to clathrate for 1 hour;
[0084] b. Add water to adjust the total amount of the solution to 6000ml, adjust the pH to 8.5 with 5% sodium hydroxide solution, add 30 grams of activated carbon (containing 0.5% of activated carbon), boil for 6 minutes, filter while hot, filter out the activated carbon, and then use 0.22 Filter with a μm microporous membrane and dilute to 6000ml with water;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com