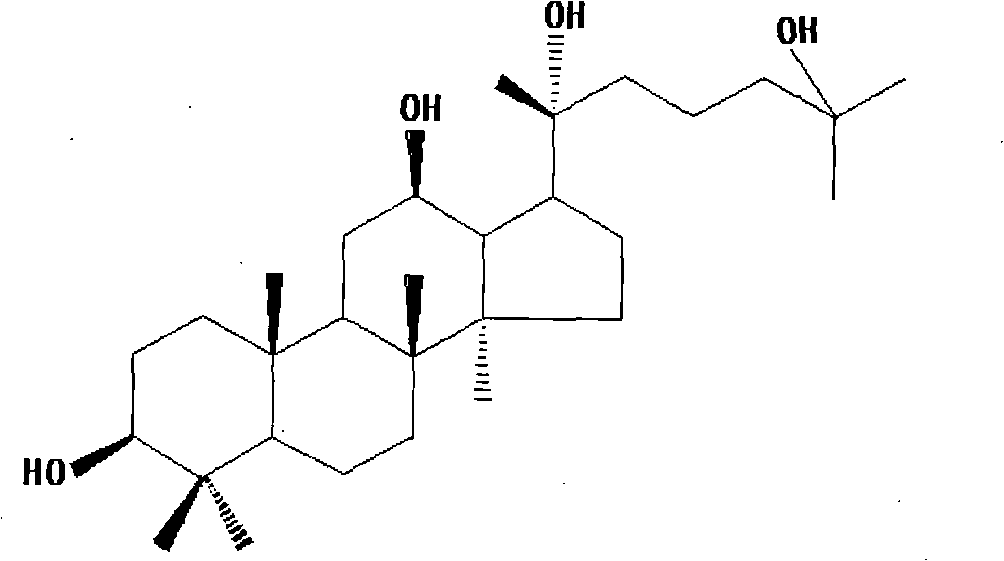
Method for preparing 20(R)-25-hydroxy-dammarane type-3beta,12beta,20-triols
A technology of -25-OH-PPD and dammarane type, which is applied in the field of preparation of 20(R)-25-hydroxy-dammarane type-3β,12β,20-triol, which can solve the difficulty of obtaining a large amount, etc. problems, to achieve strong anti-tumor activity, high purity, and stable product quality
Active Publication Date: 2011-06-15
辽宁新中现代医药有限公司
View PDF0 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, due to the difficulty in obtaining a large amount of C-K and protopanaxadiol, the preclinical evaluation of these two compounds has not been systematically reported.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a method for preparing protopanoxadiol derivatives of a formula below, namely 20(R)-25-hydroxy-dammarane type-3beta,12beta,20-triols[abbreviated as 20(R)-25-OH-PPD]. The protopanoxadiol derivatives are obtained by hydrolyzing total ginsenoside, wherein the method for hydrolyzing the total ginsenoside comprises a basic hydrolysis process, an acid hydrolysis process I and an acid hydrolysis process II. The structure of the 20(R)-25-hydroxy-dammarane type-3beta,12beta,20-triols is determined by nuclear magnetic resonance spectrum. The method is high in yield and purity, ensures stable product quality and can realize industrial production and can meet the needs for development of new antitumor medicines.
Description
Preparation method of 20(R)-25-hydroxy-dammarane type-3β,12β,20-triol This application is the application number of Liaoning Xinzhong Modern Medicine Co., Ltd. on August 7, 2006. Preparation method" divisional application. technical field The present invention relates to a preparation method of protopanaxadiol derivative 20(R)-25-hydroxyl-dammarane type-3β,12β,20-triol [20(R)-25-OH-PPD for short] . Its preparation method includes A: silica gel chromatography; B: alkali hydrolysis method, after acid neutralization, organic solvent extraction and silica gel column chromatography separation, 20(R)-25-OH-PPD white crystals are obtained; C, acid hydrolysis Method I, 20(R)-25-OH-PPD is obtained after alkali neutralization, organic solvent extraction and silica gel column chromatography separation; D, acid hydrolysis method II, add water to precipitate, wash with water until the neutral precipitate passes through the silica gel column layer After analysis and separation, white c...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07J9/00
Inventor 孙宝山赵余庆
Owner 辽宁新中现代医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com