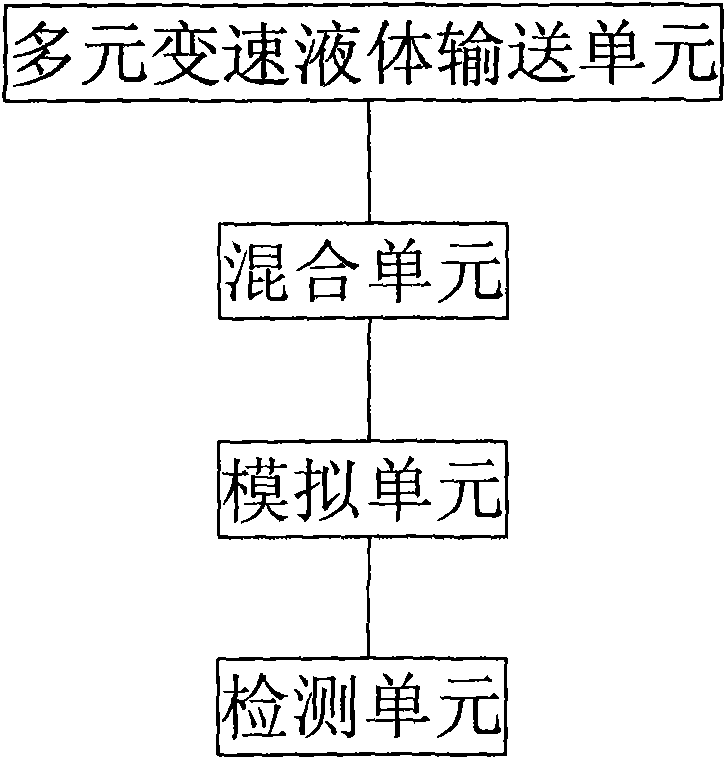
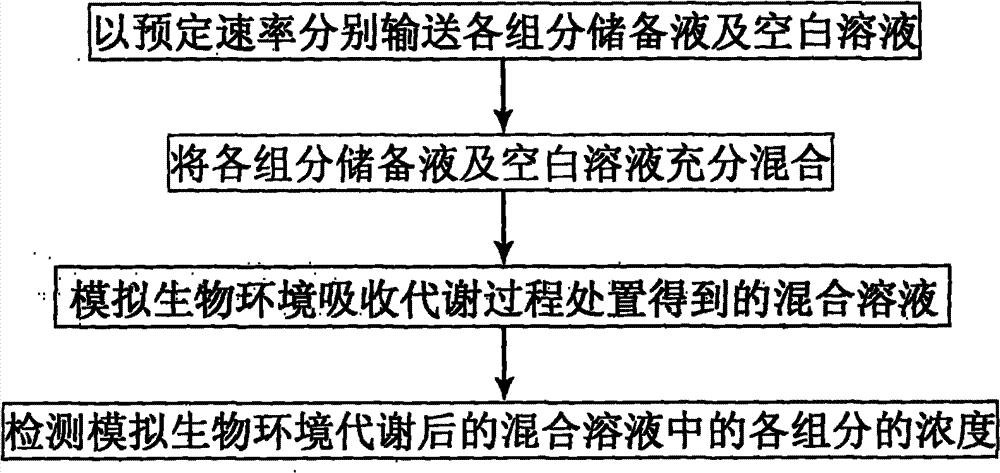
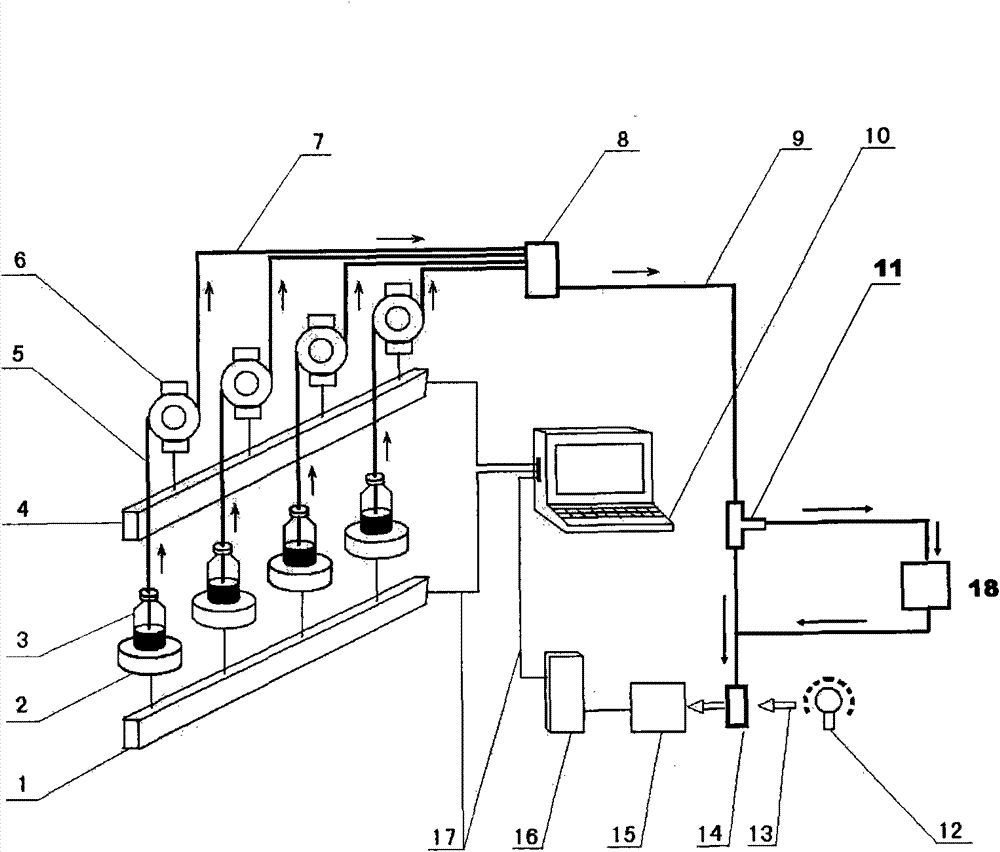
Device and method for simulating pharmacokinetics characteristics in vitro
A technology of kinetic characteristics and in vitro simulation, applied in teaching models, educational tools, instruments, etc., can solve the problems of less research on pharmacokinetic changes, no examination of component ratios, doses, and prescription screening issues, and achieve Plasma drug concentration and pharmacokinetic characteristic parameters are stable, well controllable, and easy to study the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: In vitro pharmacokinetic simulation of single-compartment model drug and single-compartment model drug combination
[0118] The pharmacokinetics of fluorouracil and uracil conform to the single-compartment model, and the blood drug concentrations of each of them injected separately are shown in Table 1.
[0119] Table 1
[0120]
[0121] In order to obtain from the device of the present invention a combined administration mixed solution of 1 times the dose of fluorouracil administered alone and 0.5 times the dose of uracil administered alone, the specific implementation methods are:
[0122] 1) Prepare a fluorouracil stock solution with 3 times the maximum concentration of fluorouracil, that is, 28.3×3=84.9 μg / mL fluorouracil solution;
[0123] 2) Prepare a fluorouracil stock solution of 0.5 times the maximum concentration of uracil, that is, 30.9 / 2×3=46.5 μg / mL uracil solution;
[0124] 3) Blank solution: water.
[0125] 4) The maximum delivery rate of...
Embodiment 2
[0130] Example 2: In vitro pharmacokinetic simulation of the combination of a single-compartment model drug and a dual-compartment model drug
[0131] The pharmacokinetics of fluorouracil conforms to the characteristics of a one-compartment model when administered by injection, and the pharmacokinetics of methotrexate conforms to the characteristics of a two-compartment model when administered by injection.
[0132] table 3
[0133]
[0134] In order to obtain a combined administration mixed solution of 1 times the individual dosage of fluorouracil and 1 times the individual dosage of methotrexate from the device of the present invention, the specific implementation method is:
[0135] 1) Prepare a fluorouracil stock solution with 3 times the maximum concentration of fluorouracil, that is, 28.3×3=84.9 μg / mL fluorouracil solution;
[0136]2) Prepare a stock solution of methotrexate with 3 times the maximum concentration of methotrexate, i.e. 150×3=450 μg / mL uracil solution;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com