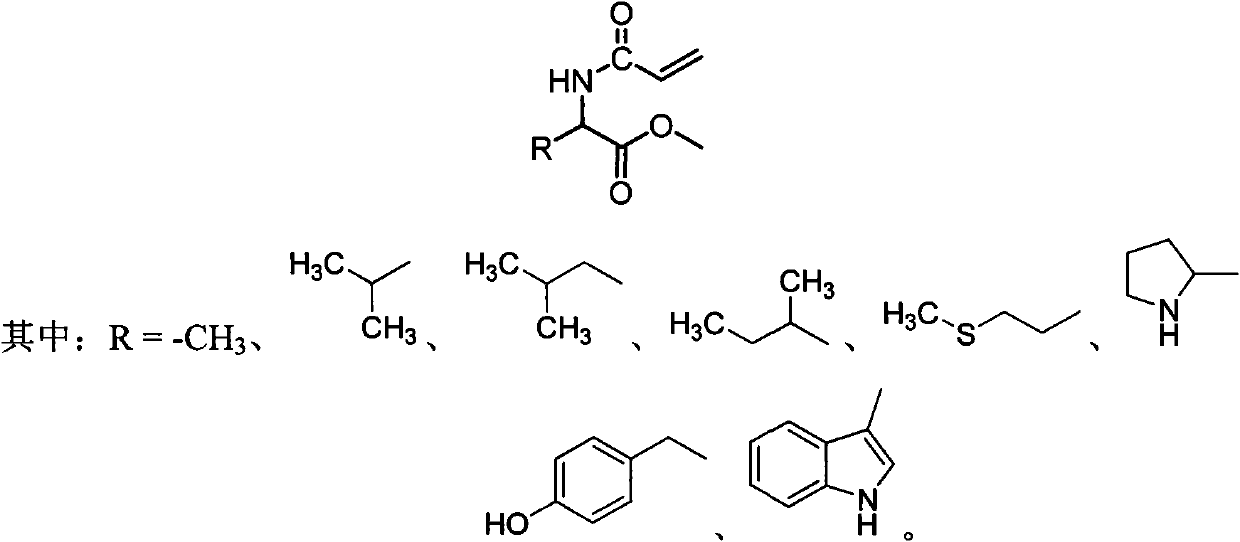
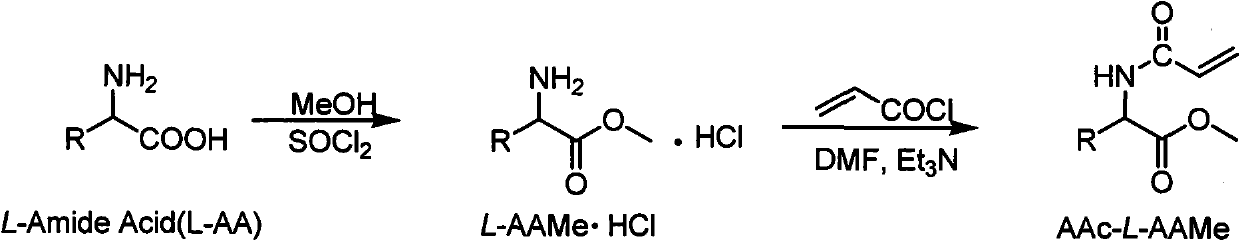
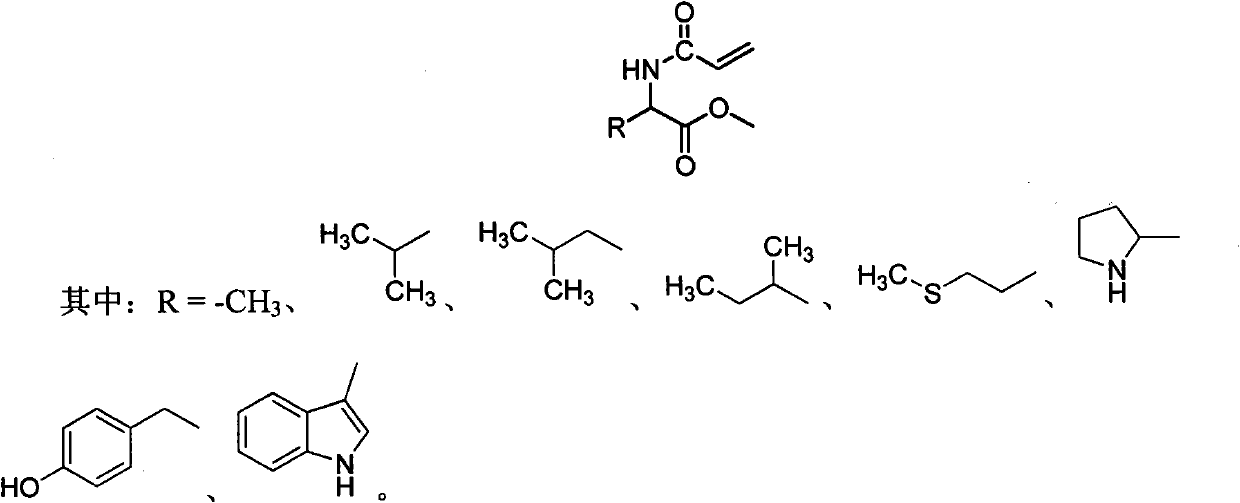
Chiral monomer containing L-amino acid group
A chiral monomer, amino acid-based technology, applied in the field of 1-substituted-2-methoxy-2-oxoethylacrylamide, can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of L-leucine chiral monomer:
[0020] Add 35mL of methanol into a 100mL three-necked flask (installed with tail gas absorption device), slowly add 3.3mL of thionyl chloride dropwise under ice bath (the temperature is controlled below 0°C), and stir for 1h. Add 5.0g of L-leucine, react at room temperature for 3h, then gradually raise the temperature to 65°C and reflux for 3h. Evaporate methanol and excess thionyl chloride, and precipitate white needle-like crystals after cooling. Suction filter and dry to obtain white needle-like L-leucine methyl ester hydrochloride.
[0021] Take 2.0g of L-leucine methyl ester hydrochloride in 15mL of N,N-dimethylformamide (DMF), and dissolve it by ultrasonic. 4 mL of triethylamine was added, and 1.13 mL of acryloyl chloride was slowly added dropwise in an ice bath. After the addition, the temperature was raised to 70°C and refluxed for 2h. After the reaction was completed, 50 mL of water was added, extracted three times ...
Embodiment 2
[0023] Preparation of L-alanine chiral monomer:
[0024] Reaction process is with embodiment 1. 1 H NMRδ (ppm): 1.21 (d, 3H), 4.31 (m, 1H), 3.58 (s, 3H), 5.53 (dd, 1H), 6.01 (d, 1H), 6.58 (d, 1H), 8.98 ( s, 1H).
Embodiment 3
[0026] Preparation of L-valine chiral monomer:
[0027] Reaction process is with embodiment 1. 1 H NMRδ (ppm): 1.21 (s, 6H), 2.49 (m, 1H), 4.31 (m, 1H), 3.48 (s, 3H), 5.03 (dd, 1H), 6.21 (d, 1H), 6.38 ( d, 1H), 9.08 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com