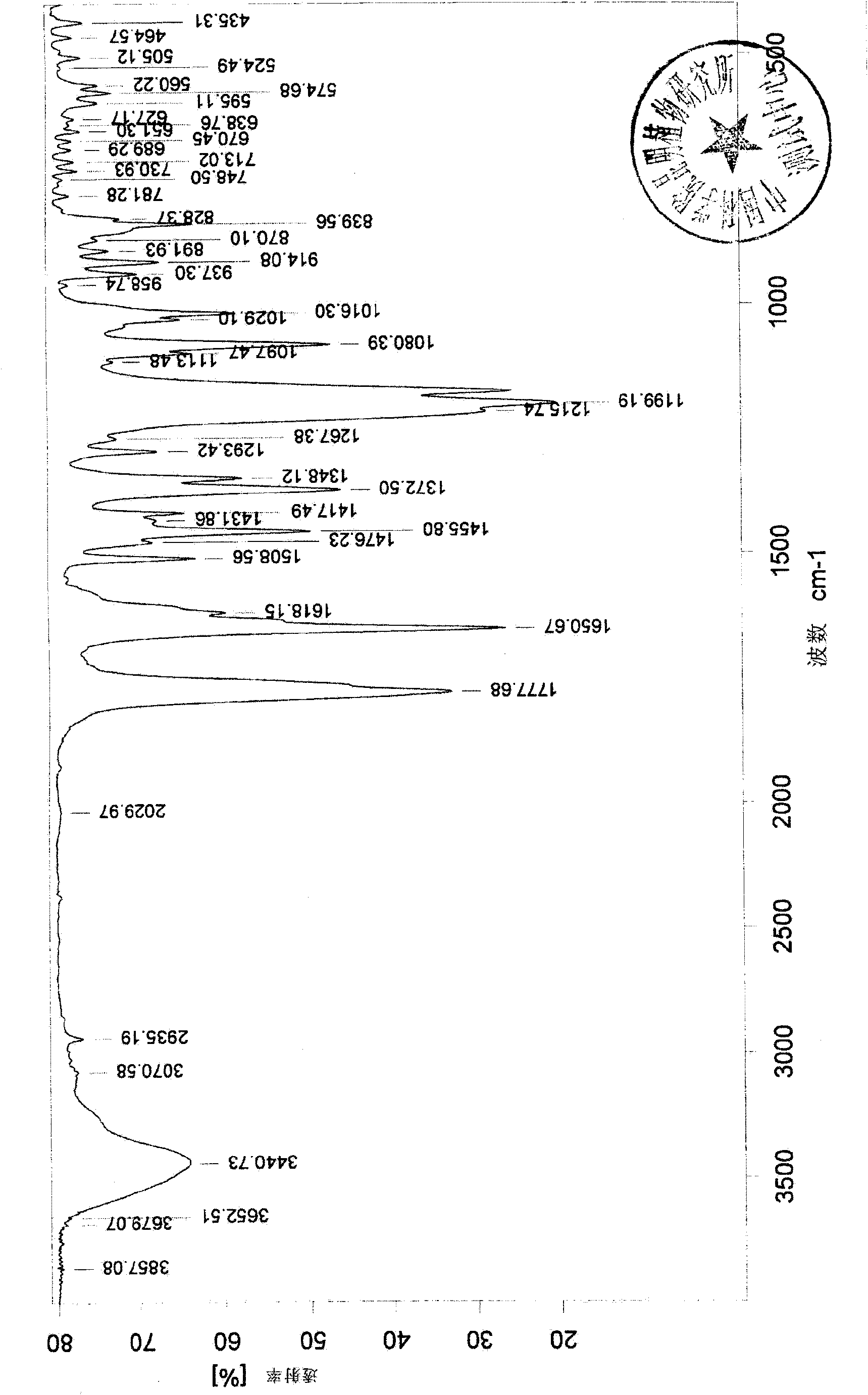
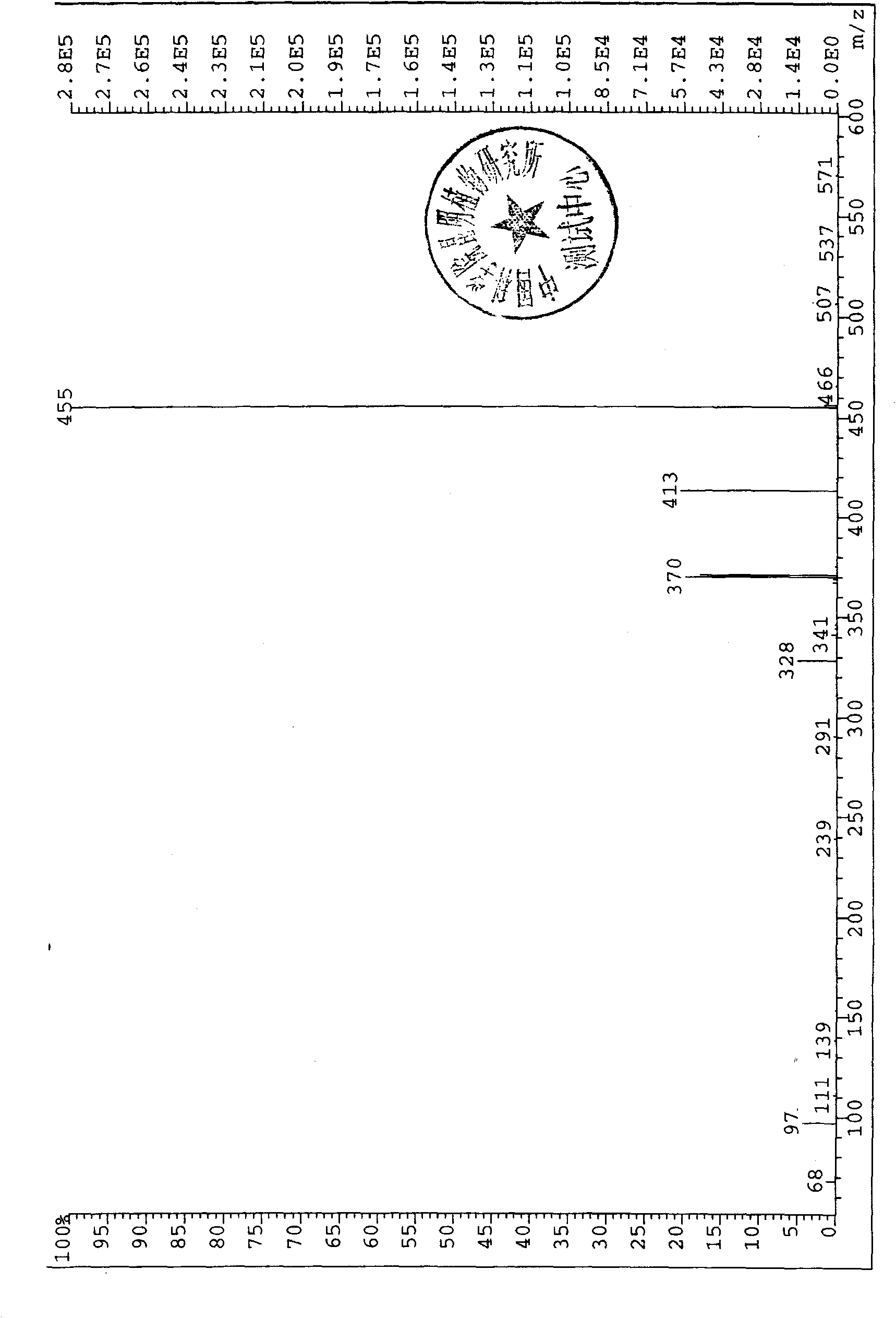
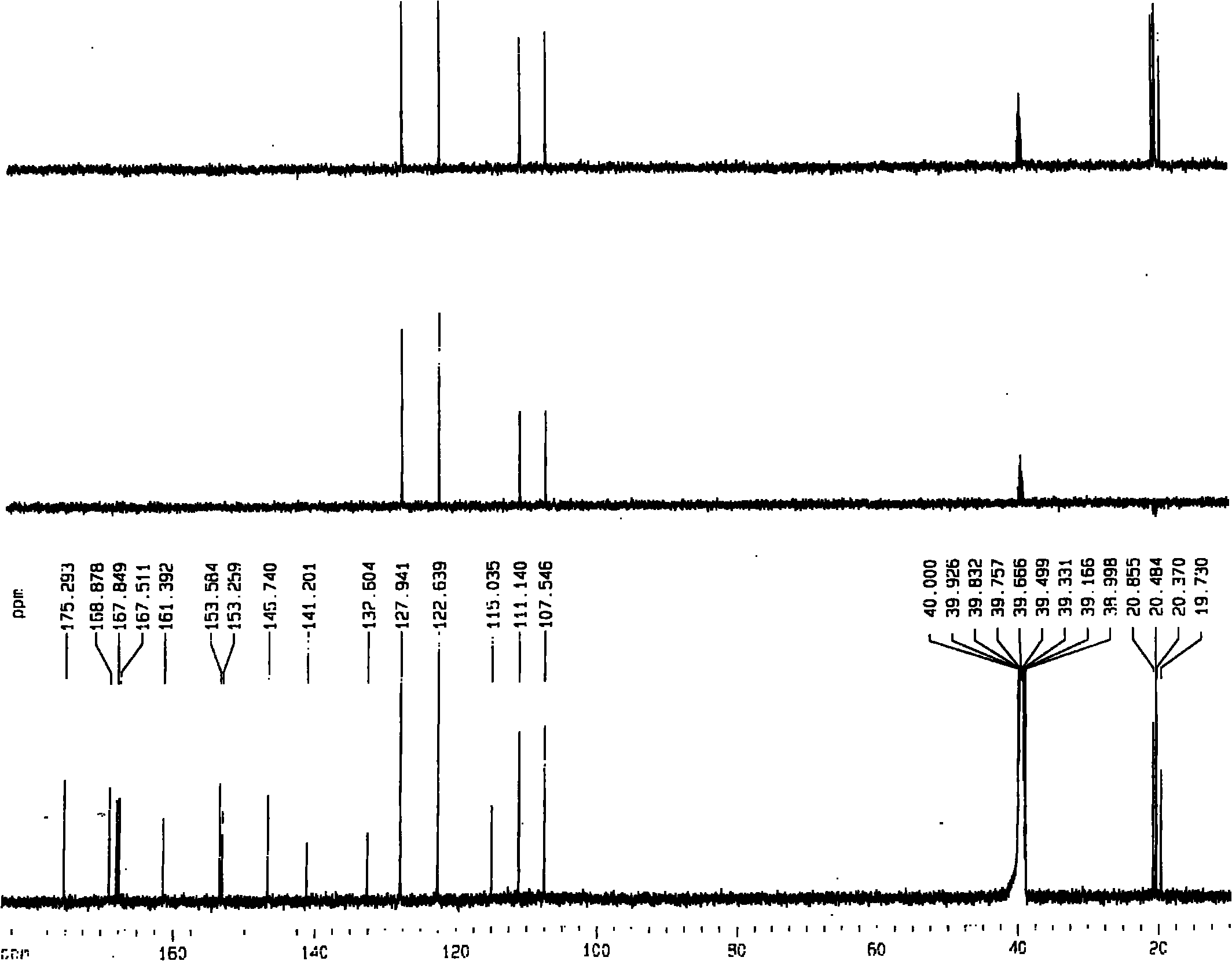
5,6,7,4'-4 acetoxy flavone and preparation method thereof
A tetraacetoxyflavone, acetylation technology, applied in the field of medicine, can solve the problems of poor stability, easy oxidation and deterioration of phenolic hydroxyl groups, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Synthesis of 5,6,7,4'-tetraacetoxyflavone
[0028] Put 10.0 g of 5,6,7,4'-tetrahydroxyflavone and 75 ml of acetic anhydride in a 300 ml Erlenmeyer flask, add 1 drop (about 0.1 ml) of concentrated hydrochloric acid or perchloric acid, and stir magnetically at room temperature for 1 hour. TLC detects that the raw material point disappears, filters and washes until neutral, and dries the filtrate to obtain 5,6,7,4'-tetraacetoxyflavone. The yield is 92%, and the purity is 98.2% as detected by HPLC.
Embodiment 2
[0029] Example 2: Synthesis of 5,6,7,4'-tetraacetoxyflavone
[0030] Put 10.0g of 5,6,7,4'-tetrahydroxyflavone and 75ml of acetic anhydride in a 300ml Erlenmeyer flask, add 10 drops of oxalic acid or triethylamine (about 1ml) dropwise, stir magnetically at room temperature for 1 hour, and detect by TLC The raw material point disappears, filter and wash with water until neutral, and dry the filtrate to obtain 5,6,7,4'-tetraacetoxyflavone. The yield is 91%, and the purity is 98.1% as detected by HPLC.
Embodiment 3
[0031] Example 3: Synthesis of 5,6,7,4'-tetraacetoxyflavone
[0032] Put 10.0 g of 5,6,7,4'-tetrahydroxyflavone and 75 ml of acetic anhydride in a 300 ml Erlenmeyer flask, add sodium hydroxide or sodium bicarbonate aqueous solution (about 10 ml) dropwise, stir magnetically at room temperature for 1 hour, and test by TLC Detect the disappearance of raw material points, filter and wash with water until neutral, and dry the filtrate to obtain 5,6,7,4'-tetraacetoxyflavone. The yield is 92%, and the purity is 98.5% as detected by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com