Quick synthesis method of reverse-2-(2-hydroxy styryl)-8-oxyquinoline zinc
A technology of hydroxystyrene and hydroxyquinoline zinc, which is applied in the direction of zinc organic compounds, chemical instruments and methods, and luminescent materials, and can solve the problems of complicated purification, cumbersome steps, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0110] The present invention will be further described below in conjunction with accompanying drawing:
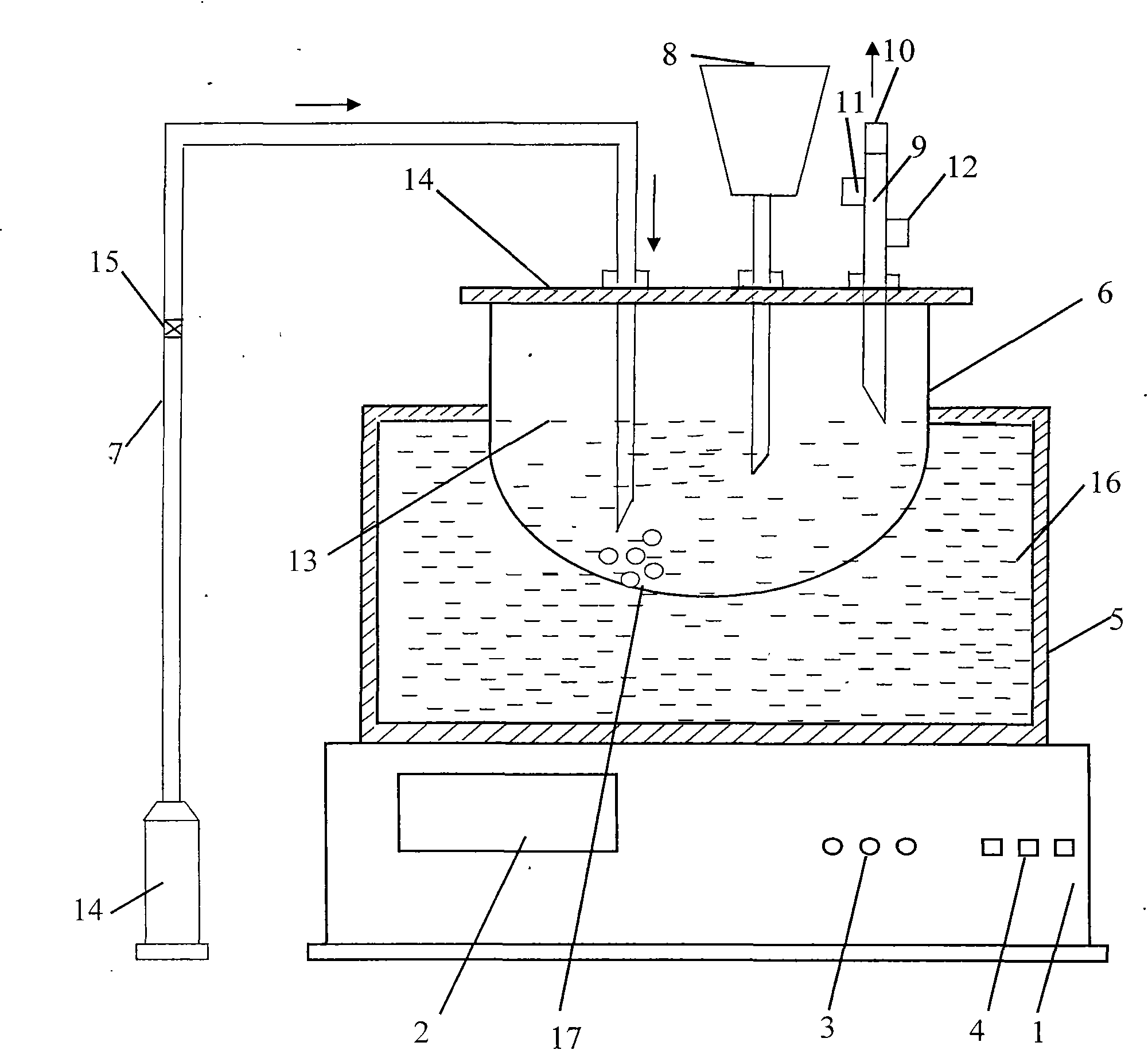
[0111] figure 1 As shown, it is the state diagram for the preparation of the final product. The position of each part must be correct, the ratio should be proportioned, and the operations should be performed in sequence.
[0112] The amount of the chemical substance required for the preparation is determined according to a preset range, with grams and milliliters as measurement units.
[0113] The three-neck flasks, beakers, stirring rods, containers, addition funnels, water circulation condensers, etc. used in the preparation should be kept clean to avoid by-products.
[0114] On the top of the stirrer 1 is an oil bath 5, in the oil bath 5 is an oil bath oil 16, on the top of the oil bath 5 is a three-necked flask 6, on the three-necked flask 6, insert a nitrogen pipe 7 and a liquid addition funnel in turn from left to right 8. Water circulation condenser pipe 9 and air ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com