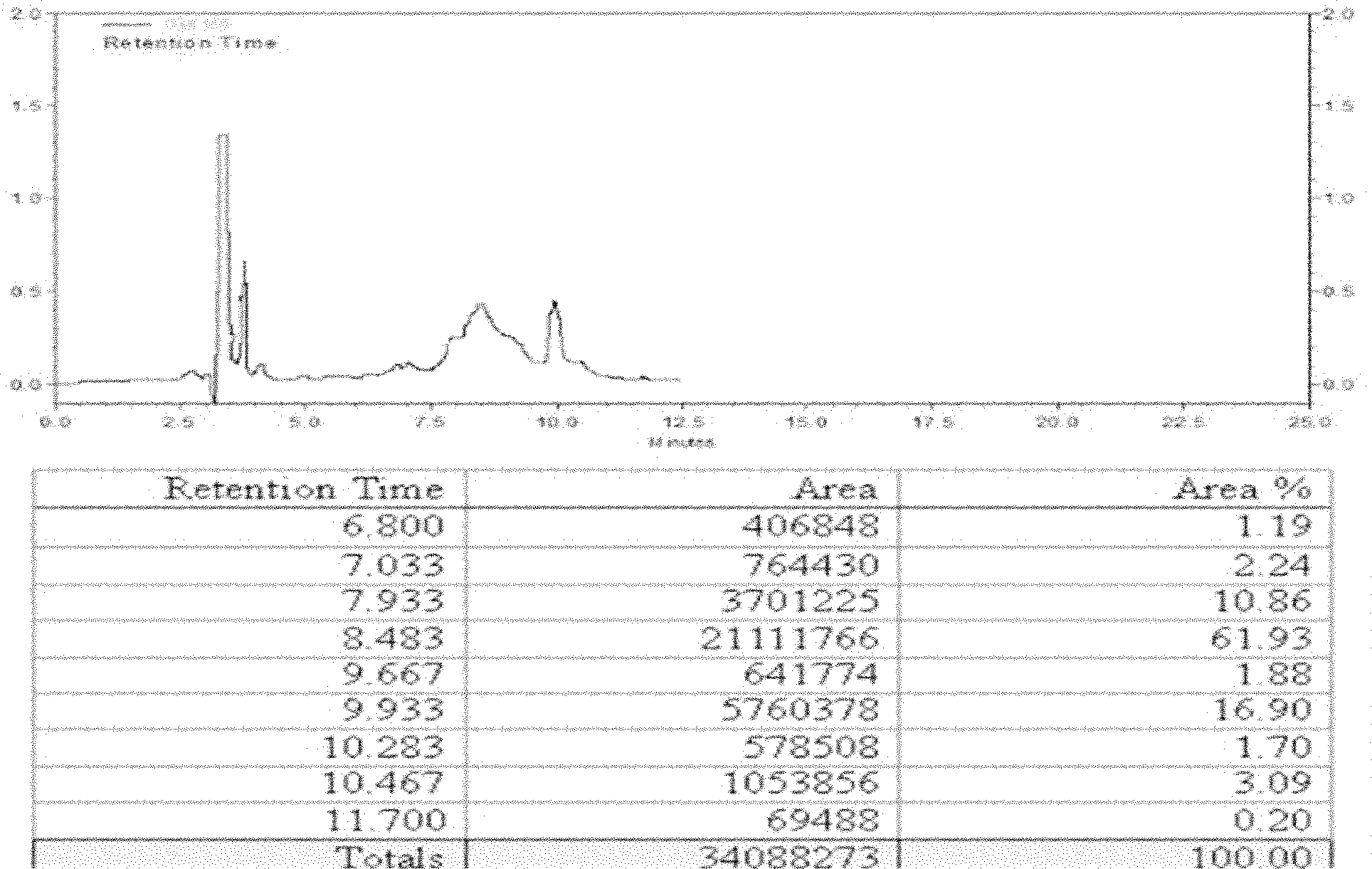
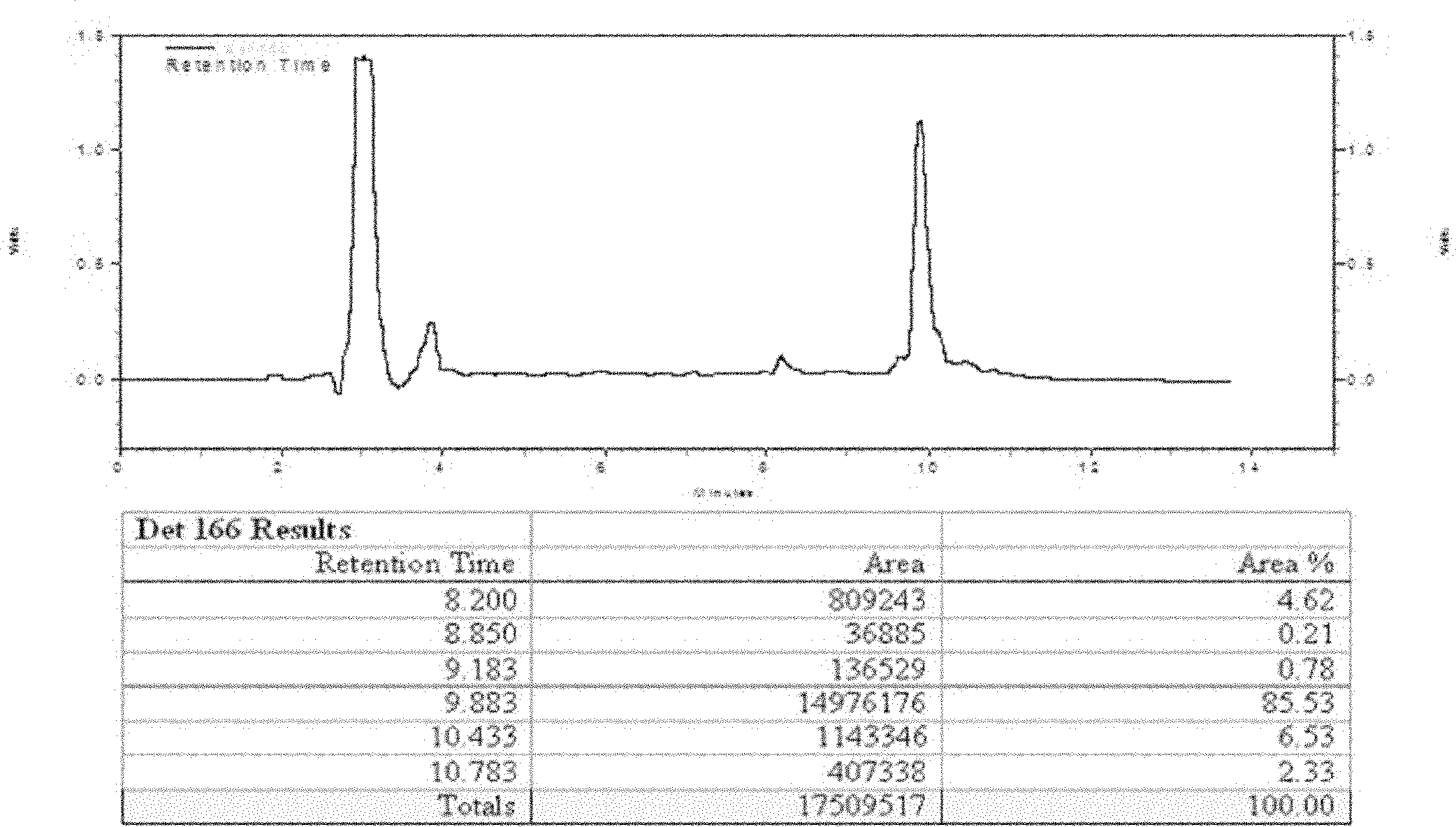
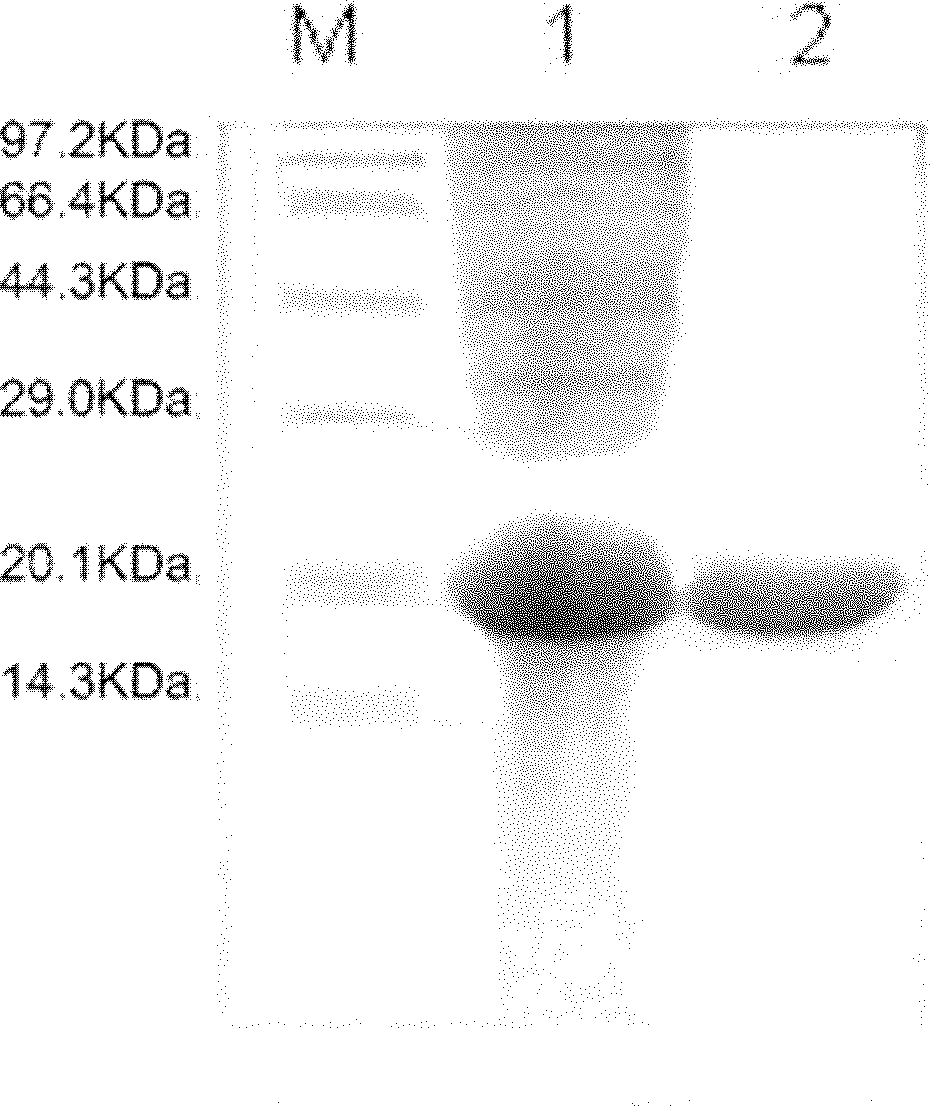Method for large-scale purification of fusion proteins containing histaq and formate hydrolysis sites
A fusion protein, large-scale technology, applied in the field of protein purification, can solve the problems of low yield and increased by-products, and achieve the effects of high yield, short time consumption and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Pretreat the fermentation broth of the engineered bacteria expressing the fusion protein containing His Taq and formic acid hydrolysis sites to obtain a crude extract of the fusion protein containing His Taq and formic acid hydrolysis sites:
[0059] 2100ml of PBS buffer with pH 7.4 and 50mM was added to 210g of wet bacterial cells (obtained from fermentation in Example 1 of Patent Application No. 201010221511.8) and mixed uniformly to obtain a bacterial cell suspension. The bacterial suspension is broken once in a high-pressure homogenizer (pressure 150MPa). The supernatant was discarded by centrifugation at 4°C at 10,000 rpm for 30 minutes, and the pellet was taken. The sample was present in the pellet as inclusion bodies. Wash the precipitate with 2100ml washing solution A (pH 8.0, 20mM Tris-HCl), and the washing method is 50rpm / min stirring for 10min; then centrifuge at 4°C 10000rpm for 30min to discard the supernatant and take the precipitate. Wash the pellet wit...
Embodiment 2
[0088] (1) Pretreating the fermentation broth of the engineered bacteria expressing the fusion protein containing His Taq and formic acid hydrolysis sites to obtain a crude extract of the fusion protein containing His Taq and formic acid hydrolysis sites: Same as step (1) in Example 1, The only difference is that the sample is 300g of bacteria obtained from fermentation in Example 2 of Patent Application No. 201010221511.8, and the volume of the solution used is 10 times the mass of the bacteria. The washed precipitate is washed with 20mM imidazole-containing dissolution buffer A (6M Urea, 50mM Na 2 HPO 4 , 500mM NaCl, pH 8.0) dissolved.
[0089] (2) Initial purification of crude fusion protein extract
[0090] A. Equilibrate the Ni-NTA column with 20mM imidazole-containing lysis buffer A (same step (1)) to stabilize the baseline, and then load the crude fusion protein extract obtained in step (1) onto the column at a flow rate of 4ml / min, Ni -The loading capacity of the NTA colum...
Embodiment 3
[0107] (1) Pretreating the fermentation broth of the engineered bacteria expressing the fusion protein containing His Taq and formic acid hydrolysis sites to obtain a crude extract of the fusion protein containing His Taq and formic acid hydrolysis sites: Same as step (1) in Example 1, The only difference is that the sample is 370 g of the bacteria obtained by fermentation in Example 3 of Patent Application No. 201010221511.8, the pressure of the high-pressure homogenizer is 120 MPa, and the volume of the solution used is 10 times the mass of the bacteria.
[0108] (2) Initial purification of crude fusion protein extract
[0109] A. Equilibrate the Ni-NTA column with 10mM imidazole-containing lysis buffer A (same step (1)) until the baseline is stable, and then load the crude fusion protein extract obtained in step (1) on the column at a flow rate of 6ml / min. -The loading capacity of the NTA column is 24.9mg protein / ml column volume; after loading the column, wash the column to bas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| salt rejection rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com