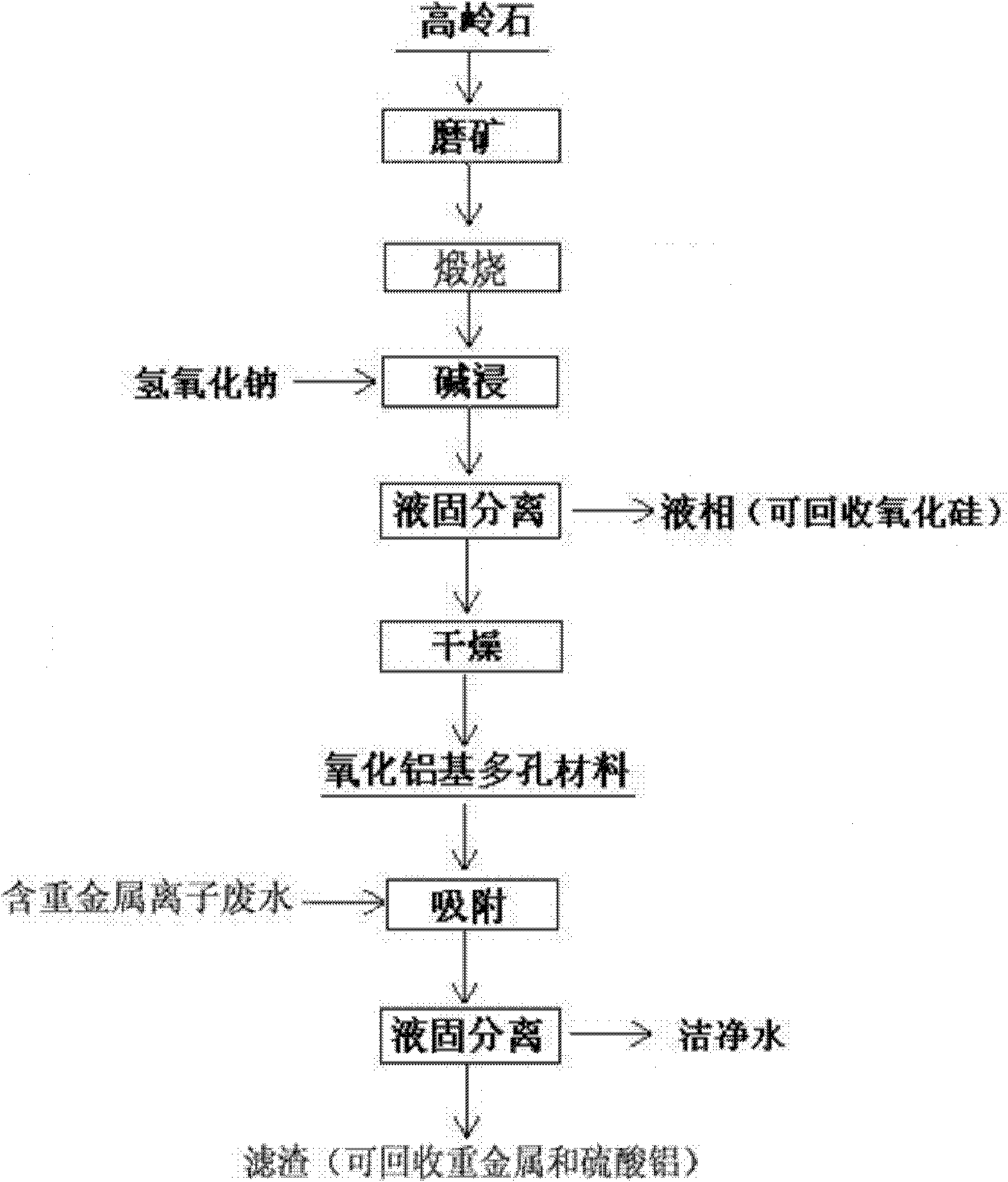
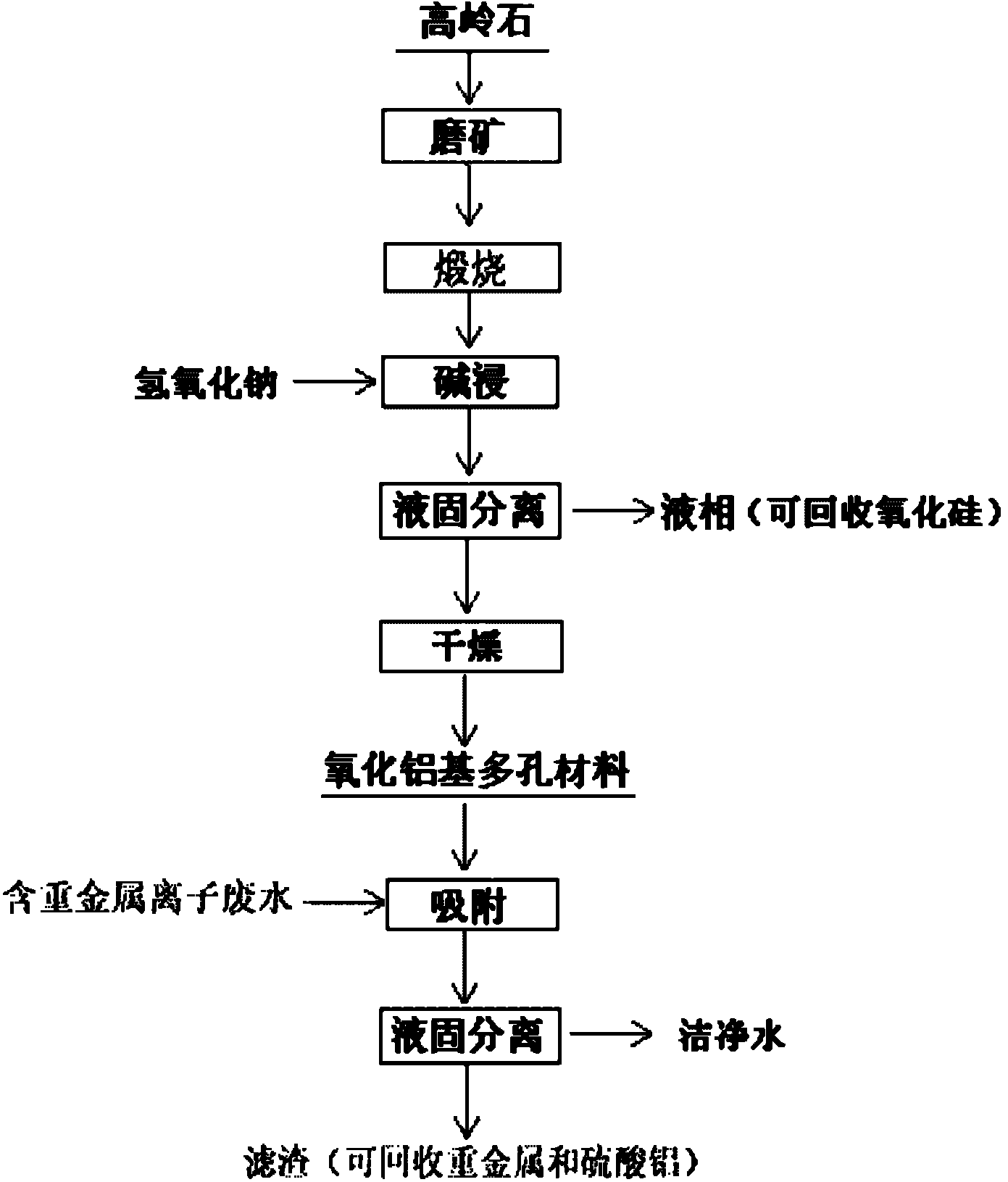
Method for preparing alumina-based porous mineral material
A technology of mineral materials and alumina, which is applied in chemical instruments and methods, other chemical processes, water/sludge/sewage treatment, etc., can solve the problems of complex adsorption material process, high cost, and insufficient adsorption capacity of heavy metal ions, etc., to achieve Wide application prospects, low cost, and excellent solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Grinding the kaolinite with a purity of 90% until the mass percentage of the particle size-100 mesh reaches 72%, the fine-grained kaolinite is calcined at 1050°C for 30min, and the calcined kaolinite and 120g / L The sodium hydroxide solution is stirred and leached with a solid-to-liquid mass ratio of 1:10. After leaching at 120°C for 90 minutes, the alumina-based porous material was obtained after solid-liquid separation and drying. The mass percentage of alumina was 70%, and the BET specific surface area was 41.1m 2 / g, the average pore diameter is 9.39nm; put the material into the heavy metal ion solution containing copper, cadmium, and lead ions with concentrations of 49.95mg / L, 50.88mg / L, and 52.03mg / L respectively, and adsorb copper at 30°C Ion 30min, cadmium ion 120min, lead ion 120min, the adsorption capacity is 22.6mg / g, 24.9mg / g, 25.8mg / g respectively.
Embodiment 2
[0021]Example 2: Grinding the kaolinite with a purity of 90% until the mass percentage of the particle size-100 mesh reaches 76%, the fine-grained kaolinite was calcined at 1100°C for 15min, and the calcined kaolinite and 120g / L The sodium hydroxide solution is stirred and leached with a solid-to-liquid mass ratio of 1:8. After leaching at 120°C for 120 minutes, the alumina-based porous material was obtained after solid-liquid separation and drying. The mass percentage of alumina was 76%, and the BET specific surface area was 45.3m 2 / g, the average pore diameter is 8.53nm; put the material into the heavy metal ion solution containing copper, cadmium and lead ions with concentrations of 49.95mg / L, 50.88mg / L and 52.03mg / L respectively, and adsorb copper at 30°C Ion 30min, cadmium ion 120min, lead ion 60min, the adsorption capacity is 22.9mg / g, 30.4mg / g, 27.5mg / g respectively.
Embodiment 3
[0022] Example 3: Grinding the kaolinite with a purity of 90% until the mass percentage content of the particle size-100 mesh size reaches 80%, the fine-grained kaolinite is calcined at 1150°C for 15min, and the calcined kaolinite and 120g / L The sodium hydroxide solution is stirred and leached with a solid-to-liquid mass ratio of 1:10. After leaching at 90°C for 90 minutes, the alumina-based porous material was obtained after solid-liquid separation and drying. The mass percentage of alumina was 79%, and the BET specific surface area was 51.4m 2 / g, the average pore diameter is 8.72nm; put the material into copper, cadmium, and lead ion concentrations of 49.95mg / L, 50.88mg / L, and 52.03mg / L respectively, and adsorb copper ions and cadmium ions at 30°C for 30min. Ion 120min, lead ion 60min, the adsorption capacity is 31.6mg / g, 54.9mg / g, 47.4mg / g respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average pore size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com