Water-soluble derivatives of edaravone, preparation method and application thereof
A technology of derivatives and synthetic methods, applied in the field of water-soluble derivatives of Edaravone and its preparation and application, can solve the problem of low oral bioavailability of Edaravone, harsh process conditions, and water-soluble problems of Edaravone. Improve insignificant problems and achieve the effect of improving drug bioavailability and water solubility
Active Publication Date: 2011-09-21
ZHONGSHAN WANHAN PHARM CO LTD
View PDF6 Cites 13 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, Chinese patent documents CN1440749A, CN1493283A and CN101288650A all report methods for preparing Edaravone freeze-dried preparations, but these methods have harsh process conditions, and the water solubility of Edaravone is not significantly improved, and Edaravone is in The problem of being easily oxidized in the solution state has not been effectively solved
In addition, since Edaravone injection is currently undergoing Phase II clinical trials for amyotrophic sclerosis in Japan, and clinical research on chronic diseases such as endothelial dysfunction may also be carried out, Edaravone has the potential for long-term use to treat the above diseases , it is obvious that long-term injection administration will bring a lot of inconvenience
Animal experiments showed that rats were given 30mg / kg orally, which was equivalent to intravenous injection of 1.5mg / kg (Edaravone (3-Methyl-1-Phenyl-2-Pyrazolin-5-one), A Novel Free Radical Scavenger, for Treatment of Cardiovascular Diseases, Recent Patents on CardiovascularDrug Discovery, 2006,1,85-93), explain that the oral bioavailability of Edaravone is extremely low, so it is necessary to transform Edaravone, obtain the equivalent and Orally available compounds for clinical selection
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
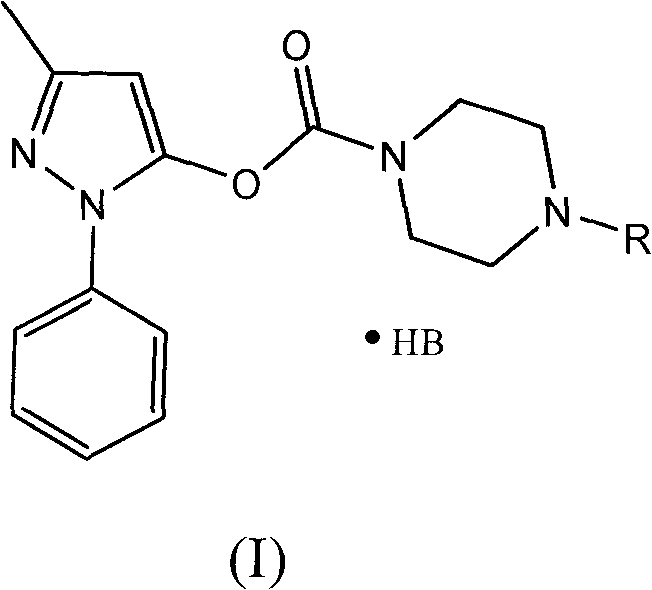
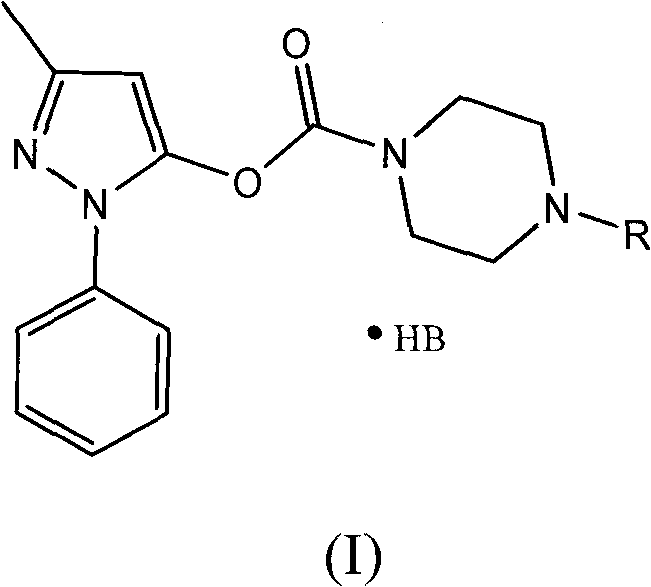
The invention discloses a series of amino carbonic ether derivatives of edaravone capable of reacting with acid salt to form water-soluble derivatives, and a preparation method thereof. The water-soluble derivatives are represented by the general formula (I), wherein R represents H, methyl, ethyl, propyl, isopropyl, butyl or benzyl, and HB represents the acid capable of reacting with organic groups containing nitrogen to form salt. The compound disclosed in the invention has favorable water-solubility and has the same cerebral protective effect in animals with edaravone.
Description
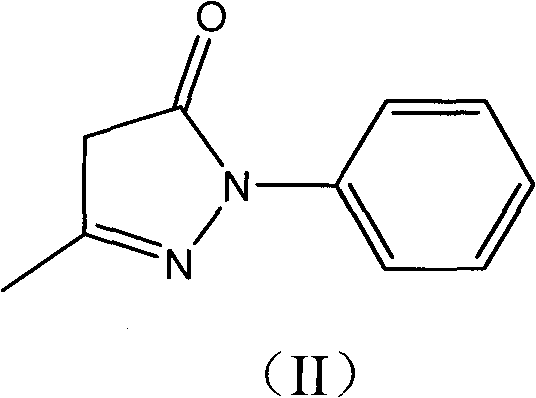
Water-soluble derivatives of edaravone and their preparation methods and applications technical field The invention relates to a series of water-soluble derivatives of edaravone and their preparation methods and applications. Background technique Edaravone is a new generation of central nervous system drug developed by Mitsubishi Corporation, Japan. It is mainly used for neurological symptoms, daily life movement disorders and dysfunction in the acute stage of cerebral infarction. It can also be used for the treatment of subarachnoid hemorrhage. . Its chemical name is: 3-methyl-1-phenyl-2-pyrazolin-5-one, and its structural formula is: Edaravone is a brain protectant (free radical scavenger). Clinical studies suggest that N-acetylaspartic acid (NAA) is a specific marker of surviving nerve cells, and its content decreases sharply in the early stage of cerebral infarction. Giving edaravone to patients in the acute stage of cerebral infarction can inhibit the reduction of...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D231/26A61K31/496A61P25/00A61P39/06A61P7/10
Inventor 李勤耕谢守全甘永军王涛陈大海高宏伟徐璐
Owner ZHONGSHAN WANHAN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com