Imaging agents of fibrotic diseases
A technology for fibrotic diseases and imaging agents, which can be used in the digestive system, in vivo radioactive agents, general/multifunctional contrast agents, etc., and can solve problems such as undescribed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
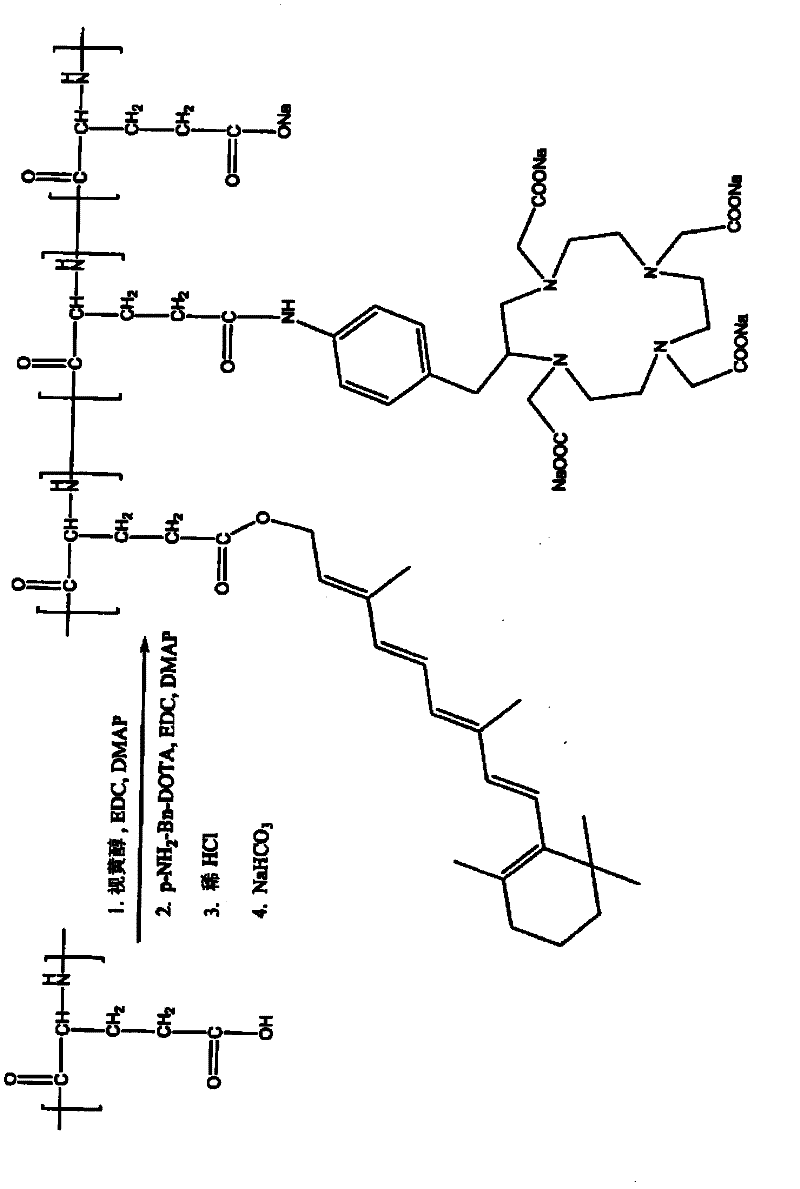
[0323] Retinoid-PGA-DOTA Synthesis
[0324] according to figure 1 The general scheme shown was to prepare the retinoid-PGA-DOTA polymer conjugate as follows: PGA (150 mg) was dissolved in DMF (15 mL). Retinol (10 mg), EDC (50 mg) and DMAP (50 mg) were added. The mixture was stirred for 24 hours. Then pNH2-Bn-DOTA (10 mg), EDC (50 mg) and DMAP (50 mg) were added. The resulting mixture was stirred for 24 hours. Dilute HCl solution (0.2M) was then added to induce precipitation. The mixture was stirred for 2 minutes and centrifuged at 10000 rpm for 15 minutes. The solid precipitate was collected, washed with water, and redissolved with sodium bicarbonate solution (0.5M). The mixture was dialyzed against water for 24 hours. The product retinoid-PGA-DOTA polymer conjugate was lyophilized. The product is identified by 1 H-MR confirmed.
Embodiment 2
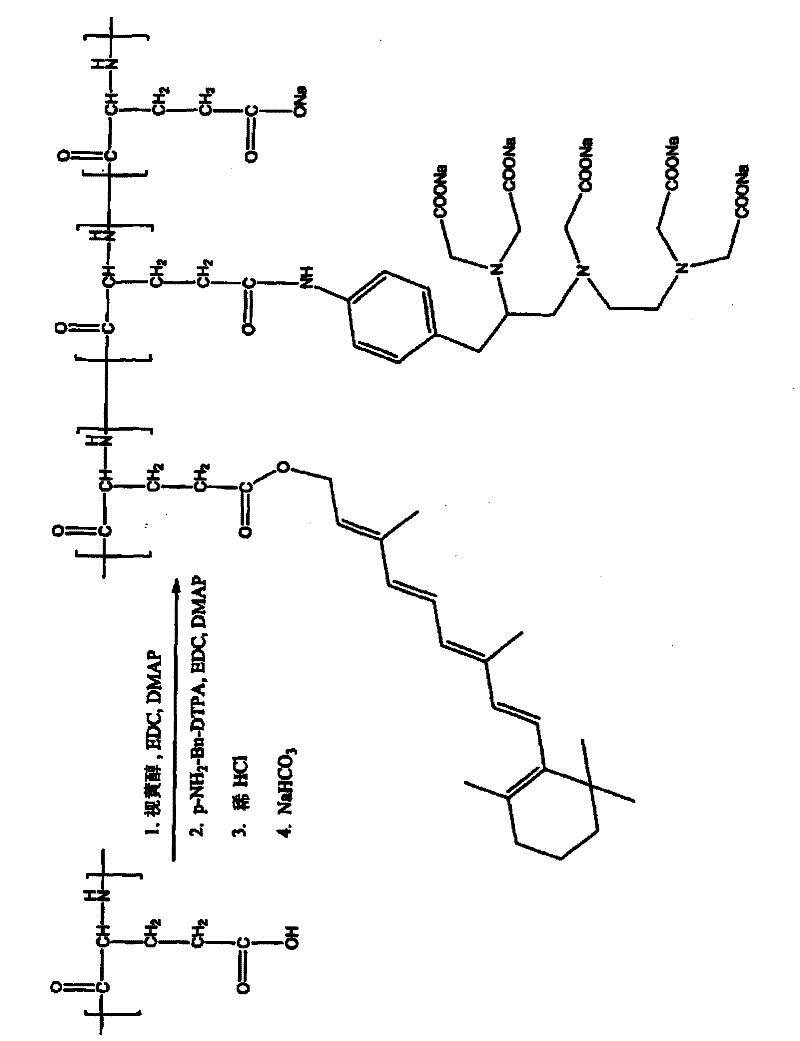
[0326] Synthesis of Retinoid-PGA-DTPA
[0327] according to figure 2 The general scheme shown was to prepare the retinoid-PGA-DTPA polymer conjugate as follows: PGA (150 mg) was dissolved in DMF (15 mL). Retinol (10 mg), EDC (50 mg) and DMAP (50 mg) were added. The mixture was stirred for 24 hours. Then add pNH 2 - Bn-DTPA (10 mg), EDC (50 mg) and DMAP (50 mg). The resulting mixture was stirred for 24 hours. Dilute HCl solution (0.2M) was then added to induce precipitation. The mixture was stirred for 2 minutes and centrifuged at 10000 rpm for 15 minutes. The solid precipitate was collected, washed with water, and redissolved with sodium bicarbonate solution (0.5M). The mixture was dialyzed against water for 24 hours. The product retinoid-PGA-DTPA polymer conjugate was lyophilized. The product is identified by 1 Confirmed by H-NMR.
Embodiment 3
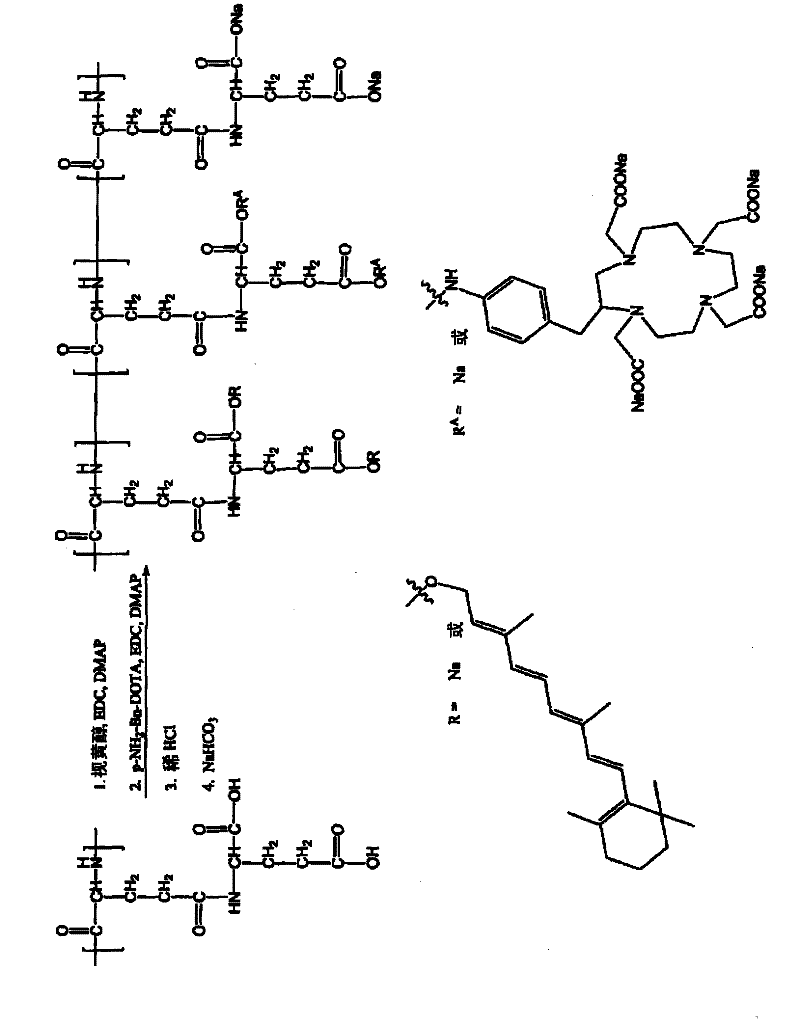
[0329] Synthesis of Retinoid-PGGA-DOTA
[0330] according to image 3 The general scheme shown was to prepare the retinoid-PGGA-DOTA polymer conjugate as follows: poly(L-γ-glutamylglutamine) (PGGA, 150 mg) was dissolved in DMF (15 mL). Retinol (10 mg), EDC (50 mg) and DMAP (50 mg) were added. The mixture was stirred for 24 hours. Then add pNH 2 - Bn-DOTA (10 mg), EDC (50 mg) and DMAP (50 mg). The resulting mixture was stirred for 24 hours. Dilute HCl solution (0.2M) was then added to induce precipitation. The mixture was stirred for 2 minutes and centrifuged at 10000 rpm for 15 minutes. The solid precipitate was collected, washed with water, and redissolved with sodium bicarbonate solution (0.5M). The mixture was dialyzed against water for 24 hours. The product retinoid-PGGA-DOTA polymer conjugate was lyophilized. The product is identified by 1 Confirmed by H-NMR.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com