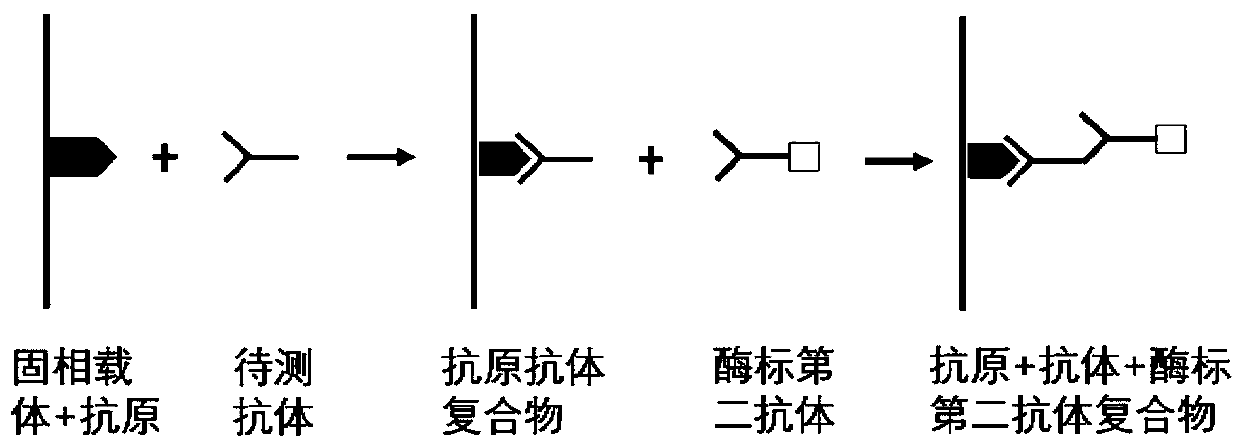
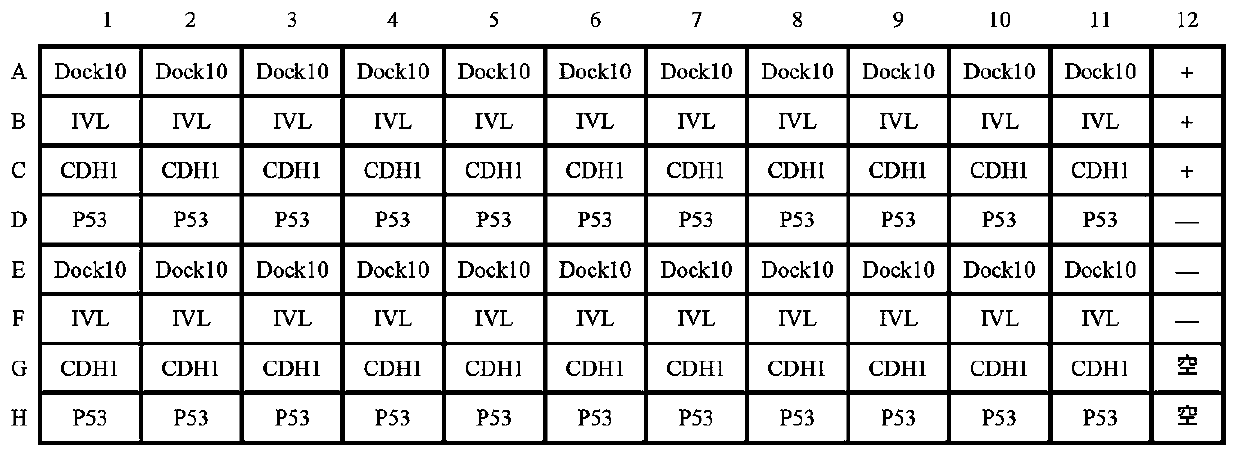
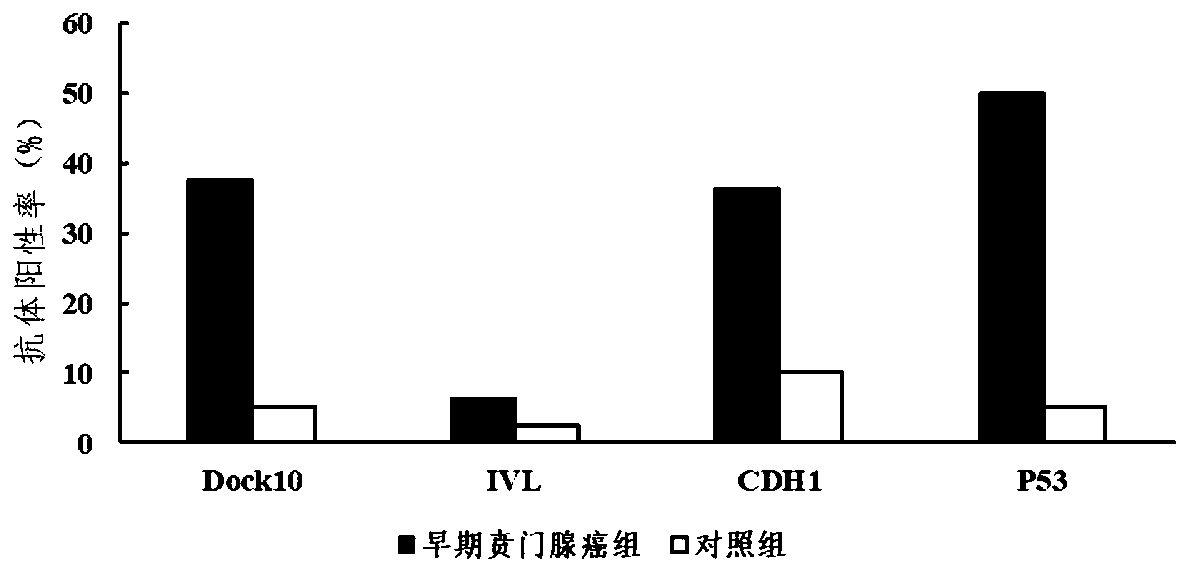
Autoantibody joint detection ELISA kit for gastric cardiac adenocarcinoma early screening
An autoantibody, combined detection technology, applied in the field of molecular biology and oncology, can solve problems such as no literature reports, achieve high sensitivity and specificity, reduce mortality, and reduce consumables.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of kit
[0028] 1. Experimental materials and reagents:
[0029] (1) 4 kinds of tumor-associated antigen proteins (Dock10, IVL, CDH1 and P53), purchased from Wuhan Aimeijie Technology Co., Ltd.;
[0030] (2) 96-well ELISA plate: 3590 (costar. USA);
[0031] (3) Coating solution: 50mM carbonate buffer solution, pH=9.6;
[0032] (4) Blocking solution: PBST buffer containing 2% (W / V) BSA;
[0033] (5) Sample diluent: PBST buffer containing 1% (W / V) BSA;
[0034] (6) Secondary antibody diluent: PBST buffer containing 1% (W / V) BSA;
[0035] (7) Enzyme-labeled secondary antibody: horseradish peroxidase-labeled RecA protein (Invitrogen);
[0036] (8) Washing solution: PBST (phosphate Tween) buffer containing 0.2% Tween 20;
[0037] (9) Positive control serum: P53 positive control serum, that is, the serum of patients with cardia adenocarcinoma who were positive for P53 antibodies by indirect ELISA and Western blot;
[0038] (10) Negative co...
Embodiment 2
[0063] Embodiment 2: the usage method of kit
[0064] 1. Serum sample incubation:
[0065] Dilute the serum sample to be tested with the sample diluent at a ratio of 1:500, and then add the diluted serum sample to the reaction wells of the 96-well microplate plate coated with antigen, with a sample volume of 100 μl / well, Place in a constant temperature incubator at 37°C and incubate for 1 h, then discard the liquid in the reaction well, and wash with washing solution 3 times, each time for 3 min.
[0066] 2. Enzyme-labeled secondary antibody incubation:
[0067] Dilute the horseradish peroxidase-labeled RecA protein with the secondary antibody diluent at a ratio of 1:40000, and then add the diluted horseradish peroxidase-labeled RecA protein to the reaction wells of the 96-well microtiter plate In this method, the sample volume was 100 μl / well, placed in a 37°C constant temperature incubator and incubated for 50 minutes, then the liquid in the sample well was discarded, and ...
Embodiment 3
[0072] Embodiment 3: Analysis of the diagnostic value of the kit of the present invention
[0073] Serum samples of patients with early cardiac adenocarcinoma and normal people were detected using the kit described in Example 1 of the present invention to evaluate and analyze the value of the kit of the present invention for screening and diagnosis of early cardiac adenocarcinoma.
[0074] 1. Sample source
[0075] A total of 160 serum samples were collected from the Henan Province Esophageal Cancer Key Open Laboratory of the First Affiliated Hospital of Zhengzhou University, including 80 normal human serum samples (control group) and 80 serum samples from early cardiac adenocarcinoma patients (early cardiac adenocarcinoma group). 80 cases of normal human serum were collected from the healthy physical examination center of the laboratory's cooperative hospital, without any tumor-related evidence. Among the 80 normal subjects, there were 43 males and 37 females, with an averag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com