Process for the preparation of substituted phenylalanines
A phenyl and hydrocarbon group technology, applied in the field of compound synthesis, can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
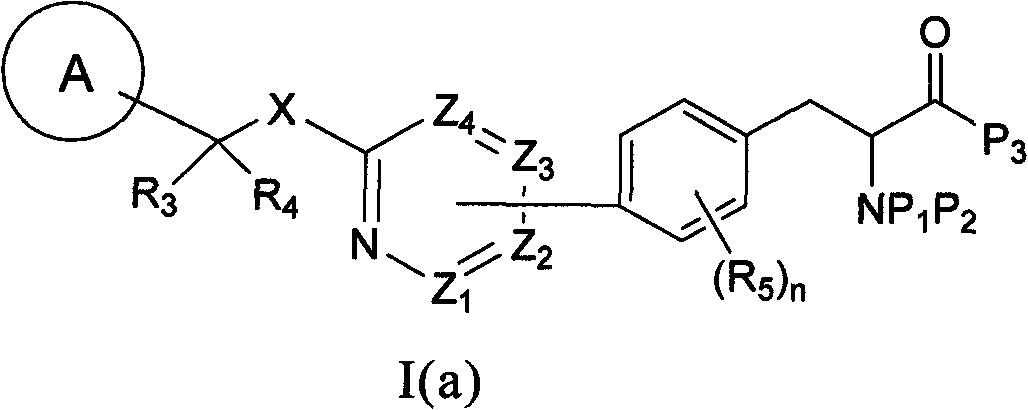
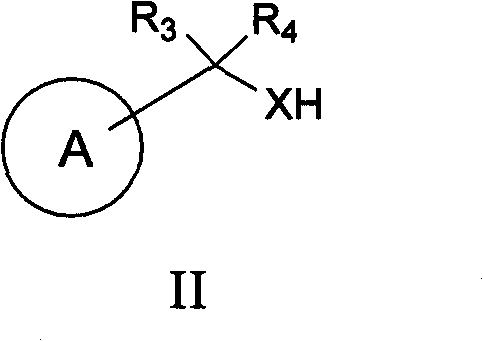
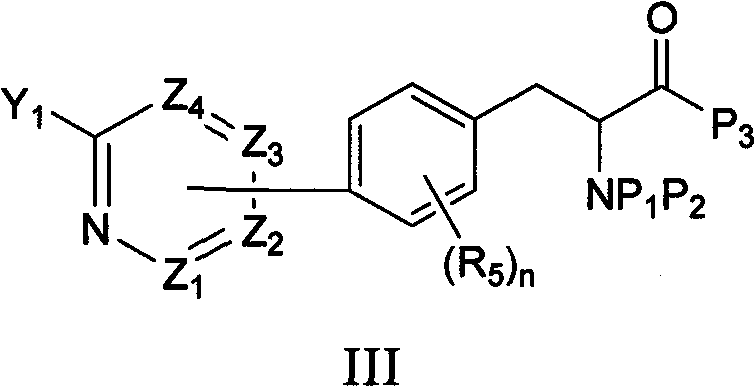
[0092] One embodiment of the invention comprises deprotecting a compound of formula I(a) to provide a compound of formula I:
[0093]
[0094] where: R 1 is hydrogen or an optionally substituted hydrocarbyl, hydrocarbyl-aryl, hydrocarbyl-heterocycle, aryl or heterocycle. In a specific embodiment, the compound of formula I is represented by formula I(b):
[0095]
[0096] where: each R 11 are independently hydrogen, cyano, nitro, halogen, OR 8 、NR 9 R 10 , or an optionally substituted hydrocarbyl, hydrocarbyl-aryl or hydrocarbyl-heterocycle; each R 12 are independently hydrogen, cyano, nitro, halogen, OR 8 、NR 9 R 10 , or an optionally substituted hydrocarbyl, hydrocarbyl-aryl or hydrocarbyl-heterocycle; m is 1-5; and p is 1-4.
[0097] In one embodiment, the compound of formula I(b) is represented by formula I(c), I(d) or I(e):
[0098]
[0099] Certain embodiments of the present invention can be understood with reference to Scheme 1:
[0100]
[0101] R...
Embodiment
[0126] The following non-limiting examples describe (S)-2-amino-3-(4-(2-amino-6-((R)-2,2,2-trifluoro-1-(3'-methanol Synthesis of oxybiphenyl-4-yl)ethoxy)pyrimidin-4-yl)phenyl)-propionic acid.
[0127] Generally, intermediate compounds 3 and 8 are first prepared as shown in Reaction Schemes 3(a) and 3(b) below.
[0128]
[0129] Scheme 3(a)
[0130]
[0131] Scheme 3(b)
[0132] An alternative synthesis of compound 8 is shown in Scheme 3(c):
[0133]
[0134] Scheme 3(c)
[0135] The intermediate is then coupled as shown in Scheme 3(d) below:
[0136]
[0137] Scheme 3(d)
[0138] In the following examples, the yields of various reactions are reported on a molar basis. Reagents are commercially available and can be purchased from Sigma-Aldrich Company, Inc. (Milwaukee, WI, USA) unless otherwise noted.
[0139] Preparation of (R)-1-(4-bromophenyl)-2,2,2-trifluoroethanol (2)
[0140]
[0141] This compound was prepared based on literature procedures (Ohkuma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com