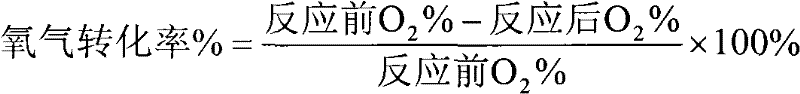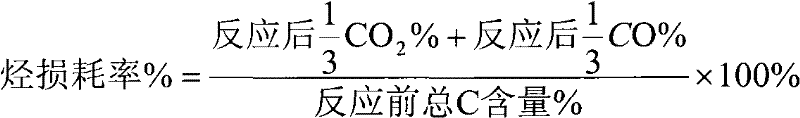Hydrogen selective oxidation catalyst used in the propane dehydrogenation process and preparation method thereof
An oxidation catalyst and propane dehydrogenation technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve problems such as low oxygen conversion rate and high hydrocarbon consumption , to achieve high oxygen conversion rate, low hydrocarbon loss rate and good technical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 2 grams over a surface of 50 meters 2 / g, nano-Al with a pore size of 29 nm 2 o 3 Carrier, add 10 ml of deionized water, pipette 0.18 ml of H 2 PtCl 6 solution and 1.44 ml of LiNO 3 solution, add dropwise 0.022 g of SnCl 4 Solution, adjust the pH value of the solution to 1.5 with 2.5% ammonia water, then soak in a water bath at 80°C for 1 hour, take out the sample and filter it, dry it in an oven at 120°C for 8 hours, and then put the sample in a muffle furnace at 550°C ℃ for 4 hours to obtain the desired catalyst. The prepared catalyst was loaded into a fixed-bed reactor, and the activity evaluation was carried out at 550°C. The results were as follows: the selectivity of hydrogen in the process was 62%, the conversion rate of oxygen was 98.1%, and the loss rate of hydrocarbon was 0.9%. .
Embodiment 2
[0028] Weigh 2 grams over a surface of 50 meters 2 / g, nano-Al with a pore size of 29 nm 2 o 3 Carrier, add 10 ml of deionized water, pipette 0.88 ml of H 2 PtCl 6 solution and 7.2 ml of LiNO 3 solution, add dropwise 0.0439 g of SnCl 4 Solution, adjust the pH value of the solution to 1.5 with 2.5% ammonia water, then soak in a water bath at 80°C for 1 hour, take out the sample and filter it, dry it in an oven at 120°C for 8 hours, and then put the sample in a muffle furnace at 550°C ℃ for 4 hours to obtain the desired catalyst. The prepared catalyst was loaded into a fixed-bed reactor, and the activity evaluation was carried out at 550°C. The results were as follows: the selectivity of hydrogen in the process was 86%, the conversion rate of oxygen was 98.5%, and the loss rate of hydrocarbon was 0.7%. .
Embodiment 3
[0030] Weigh 2 grams over a surface of 50 meters 2 / g, nano-Al with a pore size of 29 nm 2 o 3 Carrier, add 10 ml of deionized water, pipette 0.88 ml of H 2 PtCl 6 solution and 7.2 ml of LiNO 3 solution, add dropwise 0.2195 g of SnCl 4Solution, adjust the pH value of the solution to 1.5 with 2.5% ammonia water, then soak in a water bath at 80°C for 1 hour, take out the sample and filter it, dry it in an oven at 120°C for 8 hours, and then put the sample in a muffle furnace at 550°C ℃ for 4 hours to obtain the desired catalyst. The prepared catalyst was loaded into a fixed-bed reactor, and the activity evaluation was carried out at 550°C. The results were as follows: in the process, the selectivity of hydrogen was 81%, the conversion rate of oxygen was 98.2%, and the loss rate of hydrocarbon was 0.7%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com