Preparation method of ethanol Pt-Sn/Li-Al-O catalyst through acetic acid hydrogenation
A catalyst and ethanol production technology, which is applied in the direction of catalyst activation/preparation, organic compound preparation, physical/chemical process catalyst, etc., can solve the problem of low ethanol selectivity of acetic acid conversion rate, achieve low surface acid performance, improve activity and Stability and effect of improving dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The specific preparation process of the Pt-Sn / Li-Al-O catalyst in this embodiment is as follows:
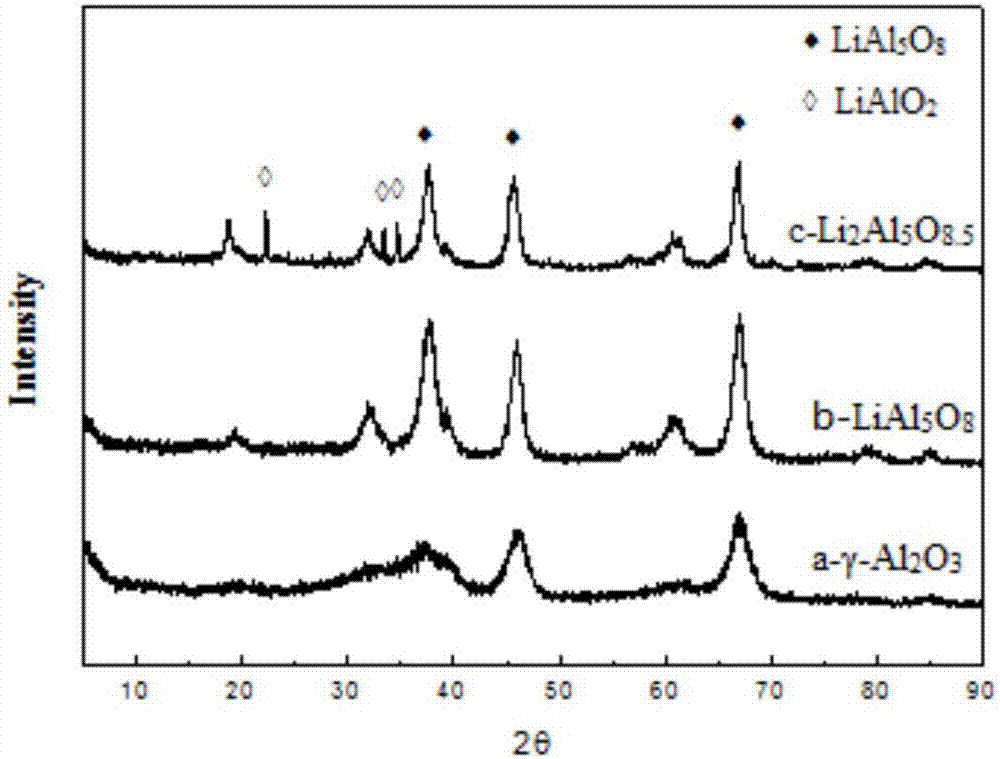
[0028] Put 1.36g of lithium nitrate solution in 50mL of deionized water, then add 10g of γ-Al 2 o 3 Stir the powder evenly, impregnate at room temperature for 2 hours, then remove water by rotary evaporation at 60°C, dry at 100°C for 12 hours, and then calcinate at 800°C for 6 hours to obtain the carrier Li 0.5 al 5 o 7.75 , wherein Li / Al=0.10.
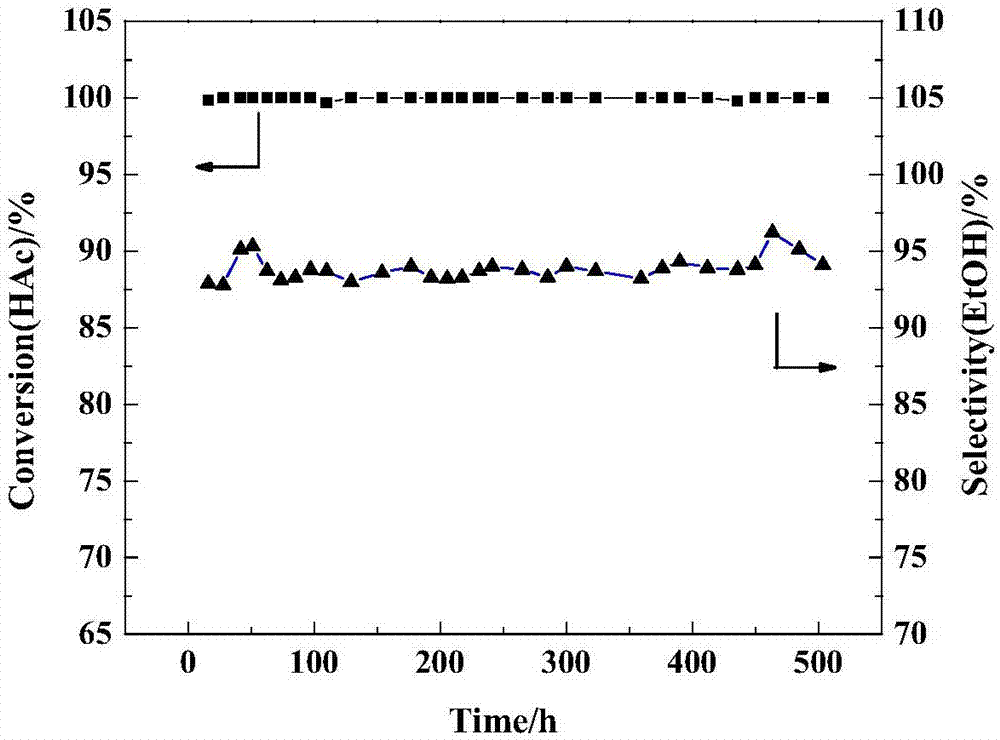
[0029]Take 5mL of chloroplatinic acid aqueous solution (0.02gPt / mL) and 0.36g of tin tetrachloride pentahydrate into 45mL of deionized water, then add the prepared carrier into the above solution and stir evenly, and soak at room temperature for 2h, then 60℃ The water was removed by rotary evaporation at a lower temperature, dried at 100° C. for 12 hours, and then calcined at 500° C. for 4 hours to obtain a catalyst powder. The catalyst powder was pressed into tablets, crushed and sieved to obtain 20-40 mesh particles, which ...
Embodiment 2
[0031] The specific preparation process of the Pt-Sn / Li-Al-O catalyst in this embodiment is as follows:
[0032] Put 2.71g of lithium nitrate solution in 50mL of deionized water, then add 10g of γ-Al 2 o 3 Particles (1-2 mm), impregnated at room temperature for 2 hours, then rotary evaporated at 60°C to remove water, dried at 100°C for 12 hours, and then calcined at 800°C for 6 hours to obtain carrier particles LiAl 5 o 8 , wherein Li / Al=0.20.
[0033] Take 5mL of chloroplatinic acid aqueous solution (0.02gPt / mL) and add it to 5mL deionized water, then add 0.36g tin tetrachloride pentahydrate to dissolve it, then add the mixed solution dropwise to the prepared carrier, wait at room temperature Volume impregnation, drying at 60°C for 24 hours, and calcination at 500°C for 4 hours to obtain catalyst particles. Recorded as catalyst 2#.
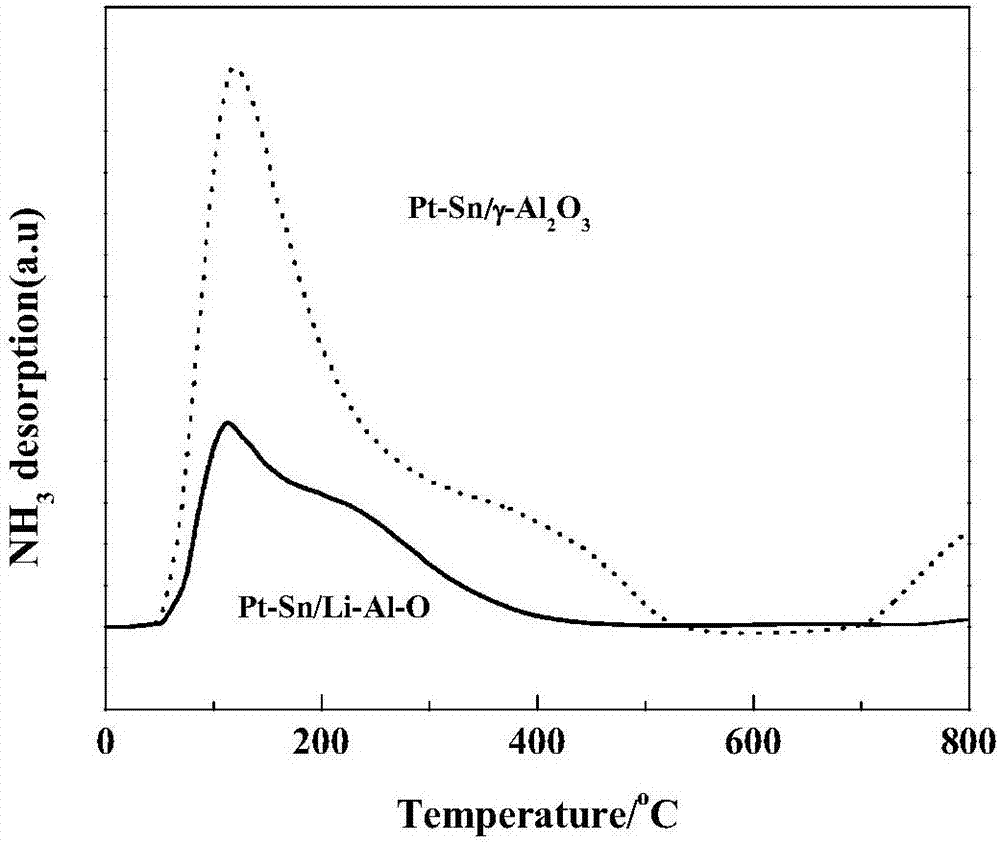
[0034] NH 3 Temperature programmed desorption (NH 3 -TPD) is an effective method for characterizing the acid strength and acid content of...
Embodiment 3
[0037] The specific preparation process of the Pt-Sn / Li-Al-O catalyst in this embodiment is as follows:
[0038] The lithium nitrate in Example 2 was changed to 4.10g, and other operating modes were unchanged, and the obtained catalyst was recorded as 3#. The carrier of catalyst 3# is LiAl 5 o 8.25 , wherein Li / Al=0.30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com