Diamine, liquid crystal aligning agent and liquid crystal display element
A technology of diamine and tetracarboxylic dianhydride, applied in instruments, luminescent materials, organic chemistry, etc., can solve the problems of reduced brightness or contrast, unmentioned ion density, narrow viewing angle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0336] Hereinafter, the present invention will be described using examples. In addition, the compounds used in the examples are shown below.
[0337]
[0338] Anhydride (1): pyromellitic dianhydride
[0339] Anhydride (19): 1,2,3,4-cyclobutanetetracarboxylic dianhydride (1,2,3,4-cyclobutanetetracarboxylic dianhydride)
[0340] Anhydride (23): 1,2,3,4-butanetetracarboxylic dianhydride
[0341] Anhydride (25): 1,2,4,5-cyclohexanetetracarboxylic dianhydride
[0342] Anhydride (37): 3,4-dicarboxy-1,2,3,4-tetrahydro-1-naphthalene succinic dianhydride (3,4-dicarboxy-1,2,3,4-tetrahydro-1-naphthalene succinic acid hydride)
[0343] Anhydride (39): 3,5,6-tricarboxy-2-carboxymethyl norbornane 2:3,5:6-dianhydride
[0344] Anhydride (49): 2,3,5-tricarboxycyclopentylacetic dianhydride
[0345] Anhydride (68): ethylenediaminetetraacetic dianhydride
[0346]
[0347] Diamine (I-1): 4-dodecyloxybenzoic acid-4-(4,6-bis(4-aminophenylamino)-1,3,5-triazin-2-ylamino)benzene ester
[034...
Synthetic example 1 2
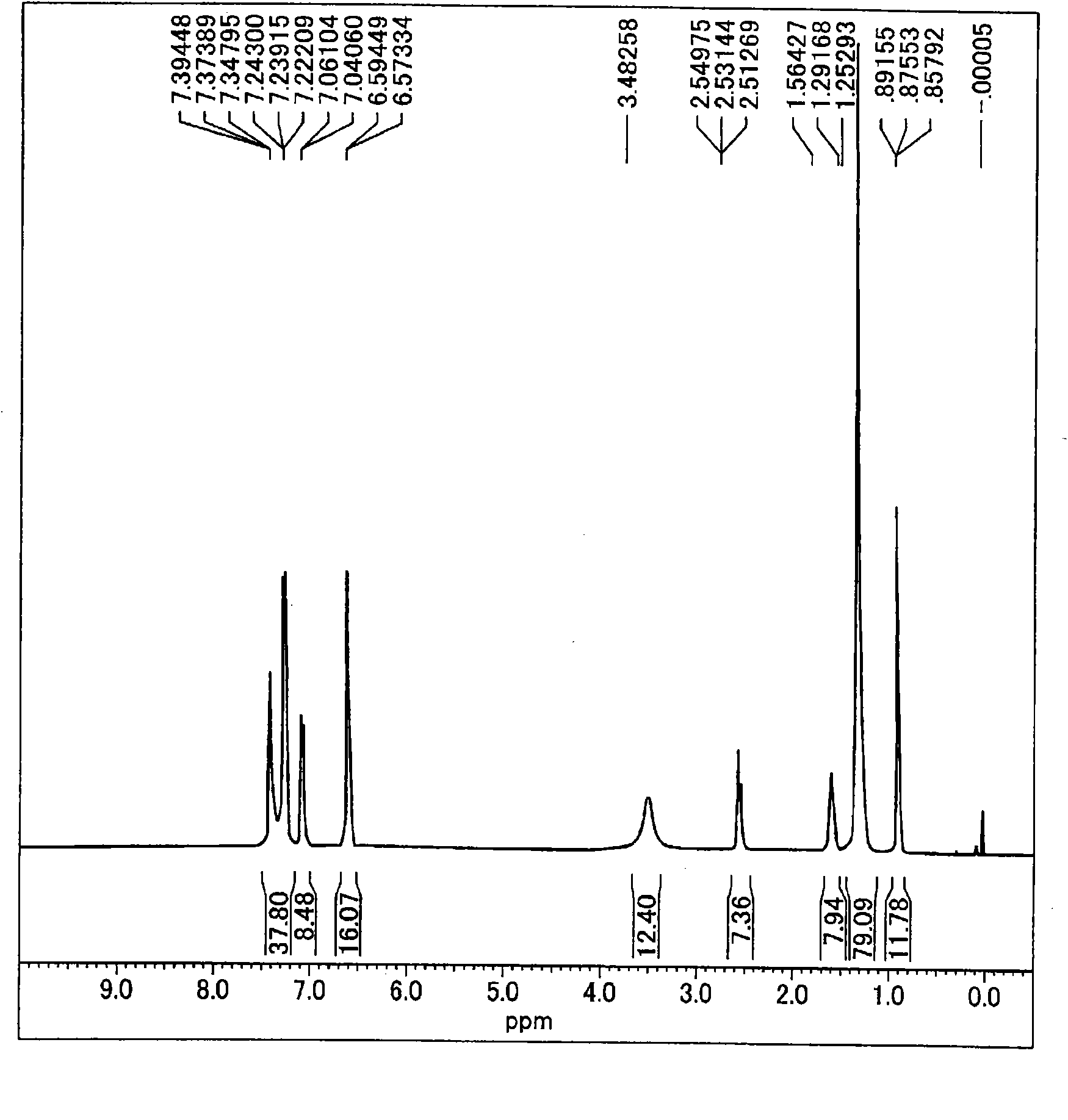
[0367] [Synthesis Example 1] Synthesis of Diamine (I-1)
[0368] [1-1] Synthesis of 4-(4,6-dichloro-1,3,5-triazin-2-ylamino)phenyl 4-dodecyloxybenzoate
[0369] 8.82 g of cyanuric chloride and 200 ml of THF were placed in the flask, and stirred while cooling to 0° C. to 5° C. in an ice bath to completely dissolve it. A solution in which 17.28 g of 4-dodecyloxybenzoic acid-4-aminophenyl ester was dissolved in 150 ml of THF was dropped therein while stirring. After completion of the dropping, stirring was continued for 2 hours while maintaining the temperature at 0°C to 5°C. Therein, a total of 2.53 g of solid sodium carbonate was dropped in a small amount each time in a manner that the temperature of the solution did not exceed 5°C. After completion of the dropping, the mixture was further stirred for 4 hours while maintaining the temperature at 0°C to 5°C. After stirring, the reaction mixture was transferred to a separatory funnel, washed three times with saturated brine, a...
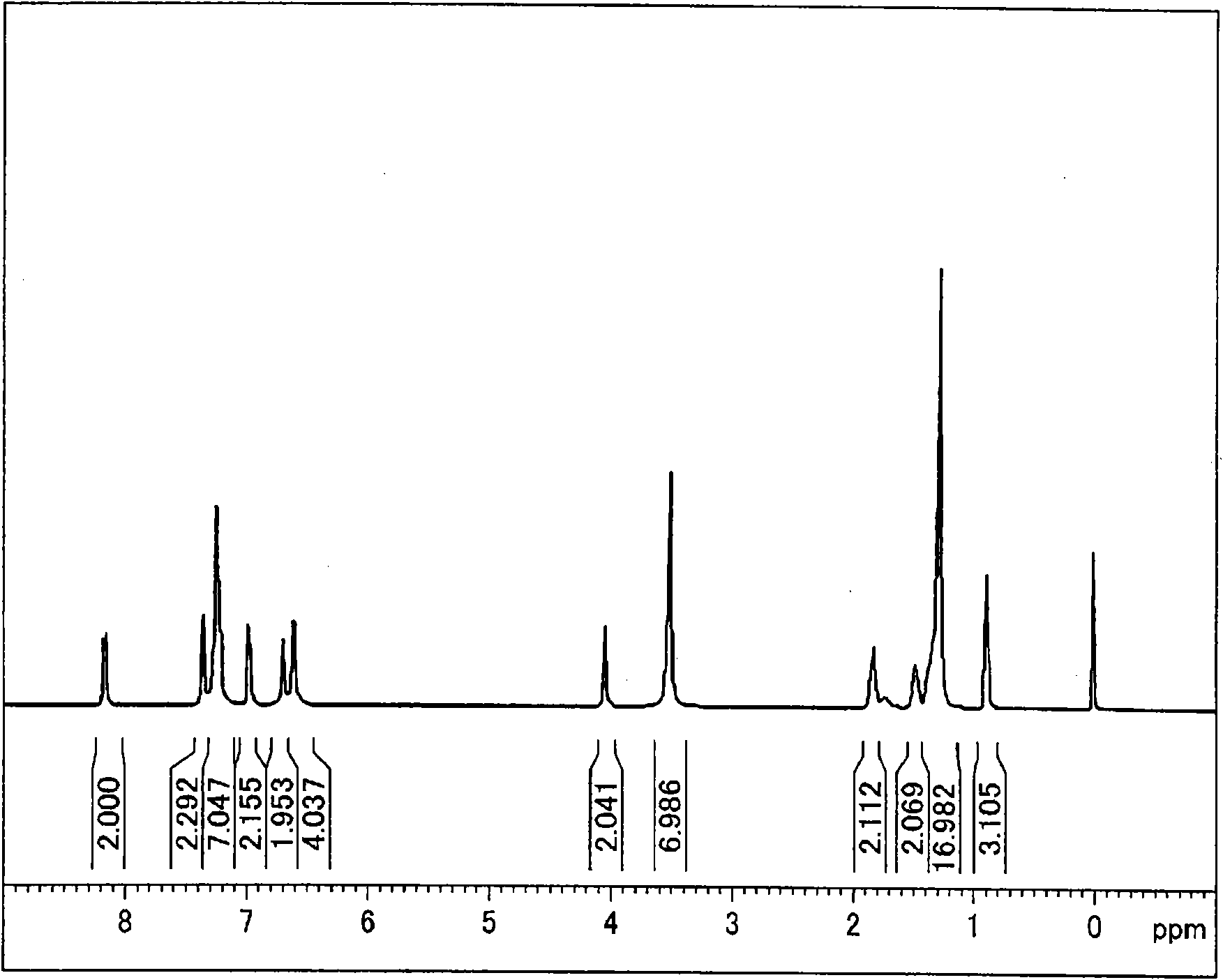
Synthetic example 2 2
[0372] [Synthesis Example 2] Synthesis of Diamine (I-2)
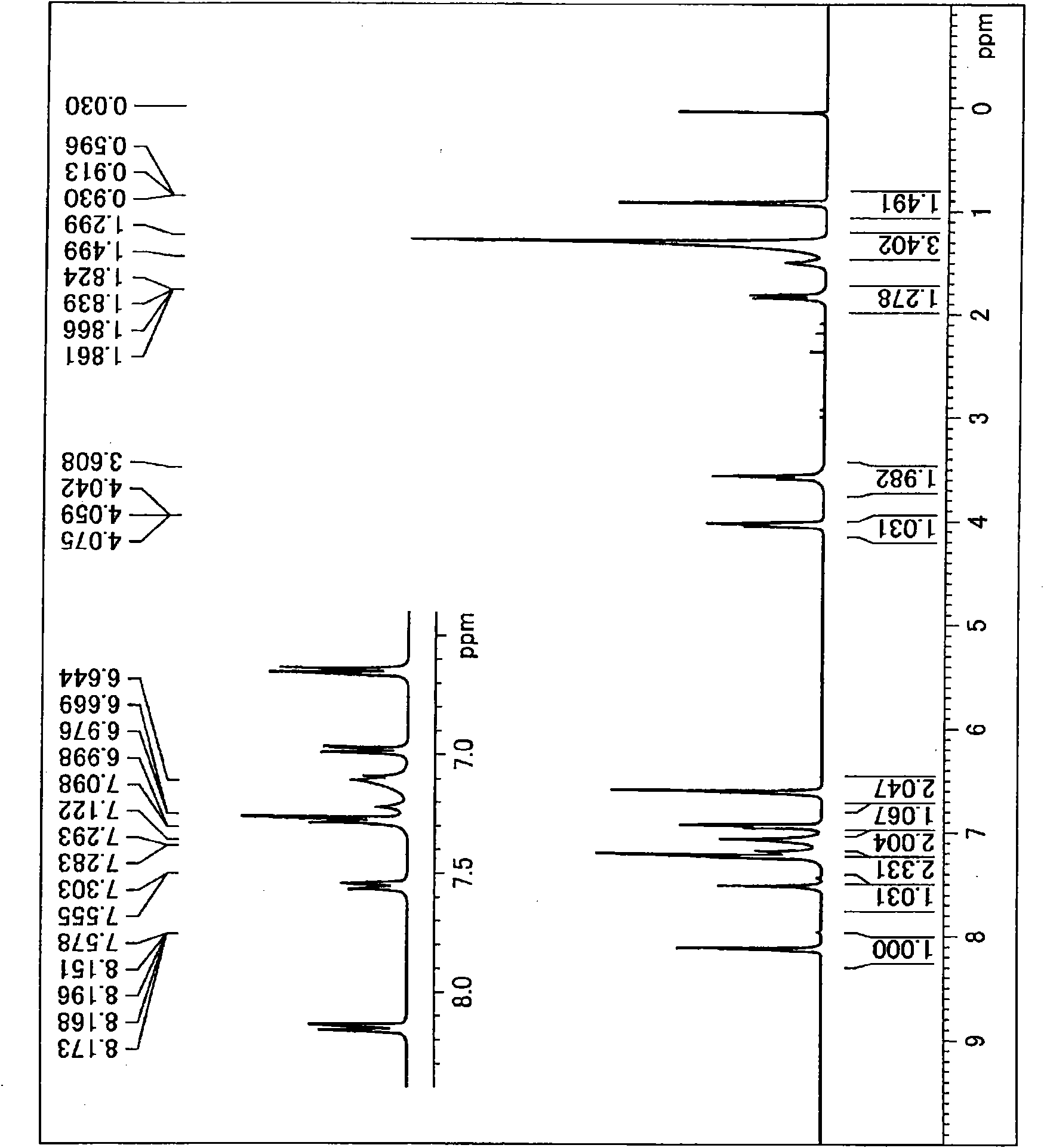
[0373] Use 4-dodecylaniline instead of 4-dodecyloxybenzoic acid-4-aminophenyl ester, in addition to carry out based on the method described in Synthesis Example 1, synthesize N2, N4-bis(4-aminophenyl )-N6-(4-dodecylphenyl)-1,3,5-triazine-2,4,6-triamine: (I-2). exist figure 2 represents the compound 1 H-NMR diagram.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com