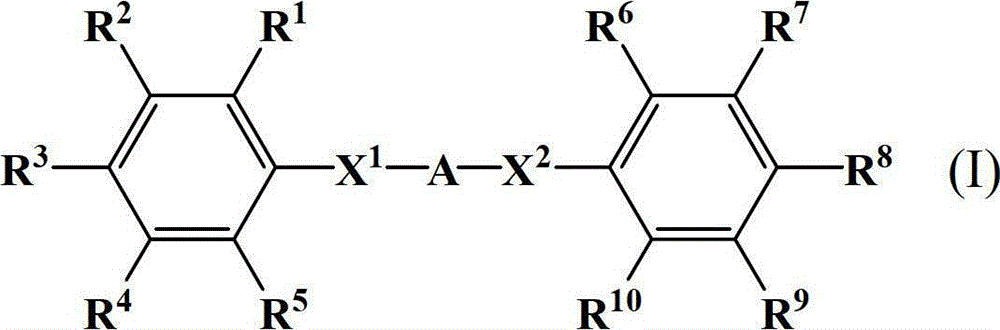
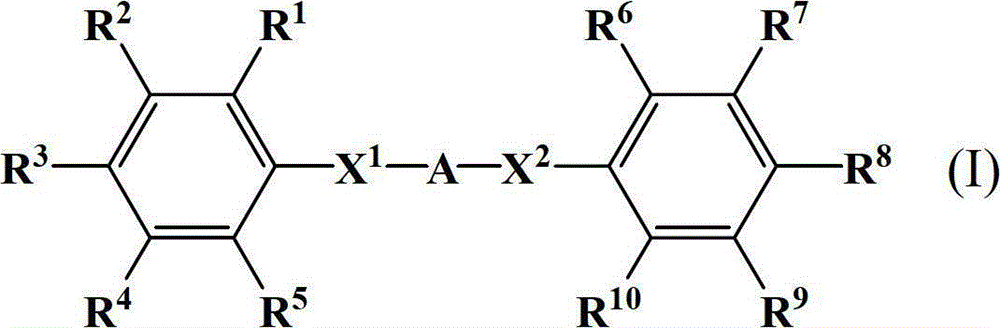
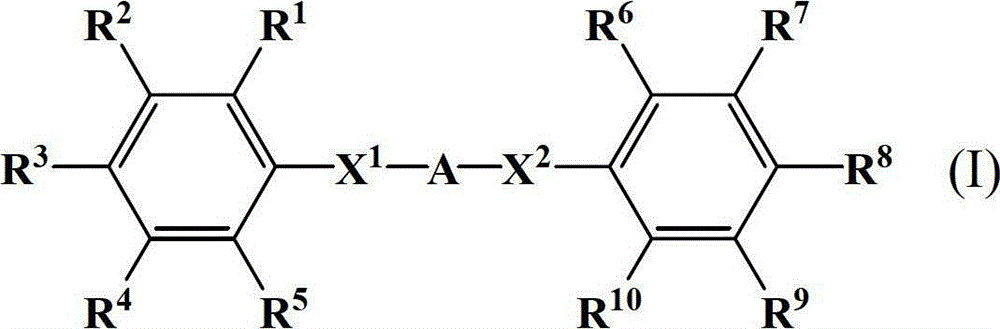
Diphenylamine compound, polyamic acid composition, polyimide composition and liquid crystal alignment agent prepared therefrom
A technology of polyamic acid and polyimide, applied in the field of diphenylamine series compounds, can solve the problem of afterimage on the screen, and achieve the effect of solving the problem of afterimage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0076] Bis(3-amino-4-methylbenzoic acid) 4,4'-(propan-2,2-diyl)bis(cyclohexyl-4,1-diyl)ester (P1)
[0077] Add 45.29g (0.25mol) of 4-methyl-3-nitrobenzoic acid (purchased from Acros) and 51.58g (0.25mol) of dicyclohexylcarbodiimide (DCC) into 288.46g of tetrahydrofuran (THF) and Mix well, and after 30 minutes, add 24.04g (0.10mol) 4,4'-(propane-2,2-diyl) dicyclohexanol (purchased from Sigma-Aldrich) and 2.44g (0.02mol) ) 4-dimethylaminopyridine (DMAP) and mix well. After reacting at room temperature for 8 hours, the filtrate was collected by filtration and concentrated to complete dryness. Then carry out recrystallization and purification 3 times with a mixed solvent of THF and isopropanol (IPA) (THF:IPA weight ratio is 4:6), and can obtain 42g bis(4-methyl -3-nitrobenzoic acid)4,4'-(propan-2,2-diyl)bis(cyclohexa-4,1-diyl)ester (I1, yield 73%) white solid.
[0078] Take 28.33g (0.05mol) of the above compound I1 and dissolve it in 152g THF, then add 2.83g Pd / C and 28.33g80% h...
Synthetic example 2
[0079] N,N'-(cyclohexyl-1,4-diyl)bis(3-amino-4-methylbenzamide) (P2)
[0080] Add 45.29g (0.25mol) 4-methyl-3-nitrobenzoic acid and 51.58g (0.25mol) DCC to 137.03gTHF and mix evenly. After 30 minutes, add 11.42g (0.10mol) 1 in sequence, 4-cyclohexanediamine (purchased from Acros) and 2.44 g (0.02 mol) of DMAP were mixed uniformly. After reacting at room temperature for 8 hours, the filtrate was collected by filtration and concentrated to complete dryness. Then recrystallize and purify 3 times with a mixed solvent of THF and n-hexane (THF:n-hexane weight ratio is 3:7), and drain the solid obtained from recrystallization to obtain 24g N,N'-(cyclohexane-1 ,4-diyl)bis(4-methyl-3-nitrobenzamide) (I2, 56% yield) white solid.
[0081] Take 22.02g (0.05mol) of the above-mentioned compound I2 and dissolve it in 114g THF, then add 2.20g of Pd / C and 22.02g of 80% hydrazine hydrate in sequence. After reacting at room temperature for 8 hours, the filtrate was collected by filtration an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com