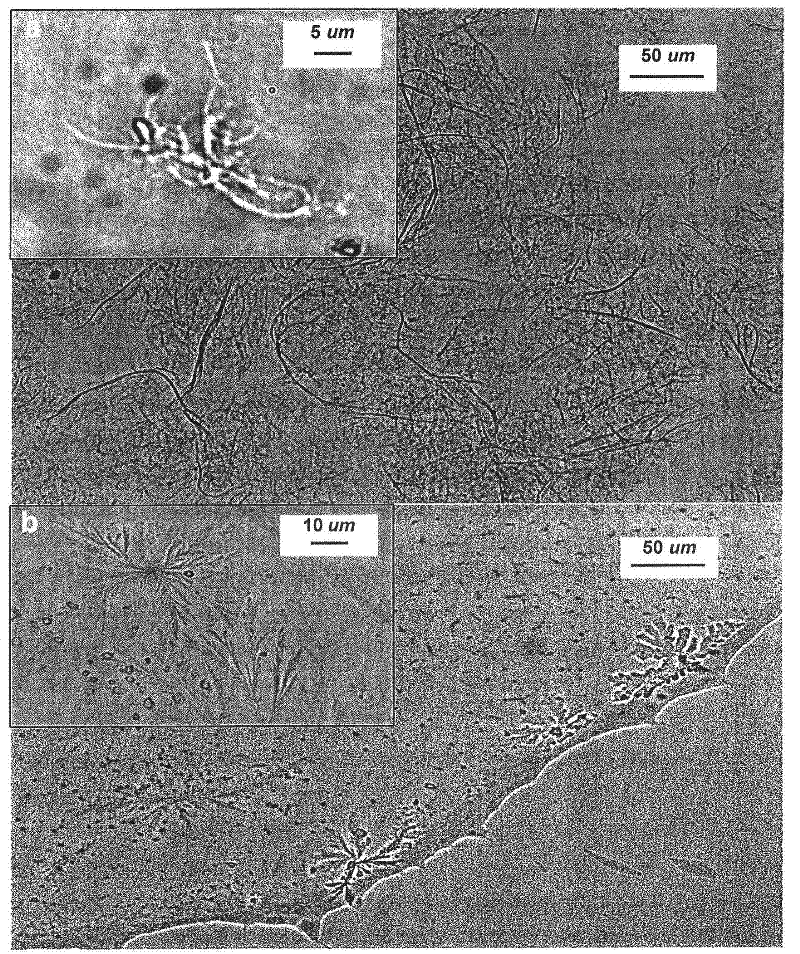
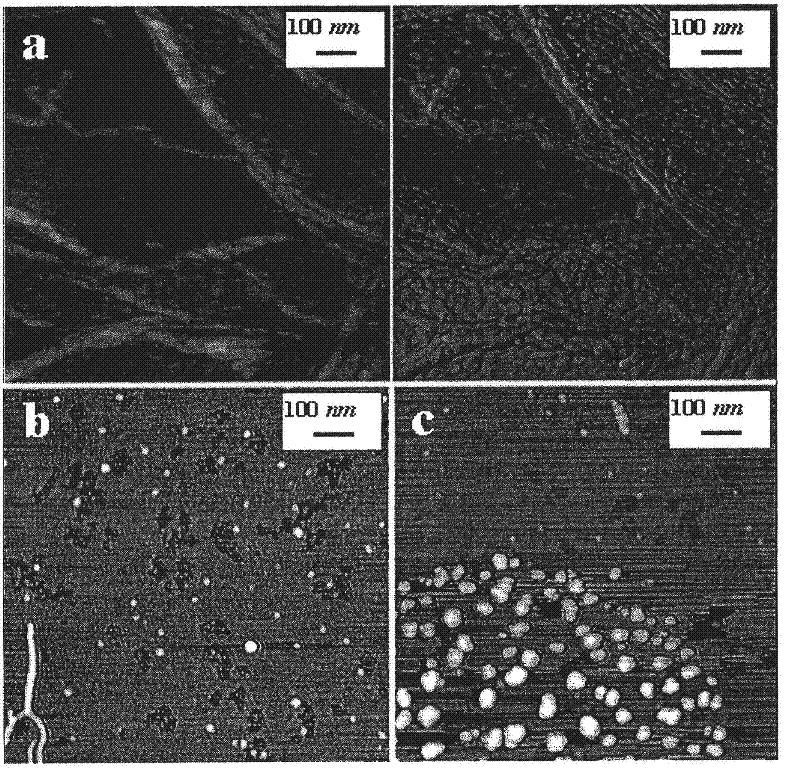
Superabsorbent polymer composite comprising a superabsorbent polymer and cellulosic nanofibrils
A technology of superabsorbent polymer and cellulose fibrils, which can be used in absorbent pads, drug delivery, medical science, etc., and can solve the problems of lack of elasticity and easy breaking.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] Example 1 - Sample S1: 0 wt% nanofibrils, comparative example (high degree of crosslinking)
[0165] Place a cooling bath (cold water + ice) on top of a magnetic stirrer. 5.24g NaOH (67% acid neutralized) was dissolved in 17.009g Ultra Pure Elga water. A bottle was charged with 13.255g Ultra Pure Elga water, 13.8g acrylic acid monomer (0.192 moles) and placed in a cooling tank. After mixing for 10 minutes, NaOH solution was added dropwise to the bottle. The bottle was placed in a water tank pre-set to 42°C and 0.242 g of crosslinker MBA (0.85 mole % per mole of monomer) was added, stirring speed was increased to ensure that the powder was added to the solution. After 10 minutes, initiator VA-044 (0.1 mole % of monomer, 0.059 g) was added by syringe as a 10% solution in Ultra Pure Elga water. The temperature of the tank was raised to 50°C and the reaction was allowed to proceed at 50°C. Ten minutes after the network had formed, the bottle was sealed, the heat source wa...
Embodiment 2
[0166] Example 2 - Sample S2: 12% by weight fibrils per weight of acrylic monomer (low degree of crosslinking)
[0167] To increase the concentration of nanofibrils in the solution, a certain amount of nanofibril solution (1.5% in deionized water) was filtered to remove part of the water. The nanofibrils will still be wet after this step and contain water.
[0168] 17.863 g of the nanofibril solution (0.62 g of nanofibrils) was charged to the E flask, and the flask was then placed in a cold water bath. 3.705 g NaOH (67% acid neutralized) was added to flask E in small increments and the solution was mixed until all NaOH particles were dissolved.
[0169] Place a sink of cold water (cold water + ice) on top of a magnetic stirrer. The nanofiber-NaOH solution was transferred into a bottle, then 19.5 g of the nanofibril solution (0.60 g of nanofibrils) was added and the bottle was placed in a sink of cold water. The solution was mixed for approximately 15 minutes, after which 10...
Embodiment 3
[0171] Example 3 - Sample S3: 0% by weight nanofibrils per weight of acrylic monomer, comparative example (low degree of crosslinking)
[0172] Place a sink of cold water (cold water + ice) on top of a magnetic stirrer. The bottle was filled with 25.53g Ultra Pure Elga water, 1Og acrylic acid monomer (0.139 moles) and placed in a cooling tank. 14.98 g of NaOH solution (25% solution in Ultra Pure Elga water, 67% neutralized acid groups) was added dropwise to the bottle.
[0173] The bottle was placed in a water tank pre-set to 42°C and 0.089 g of crosslinker MBA (0.42 mole % per mole of monomer) was added, stirring speed was increased to ensure that the powder was added to the solution. After 10 minutes, initiator VA-044 (0.1 mole % per mole of monomer, 0.045 g) was added by syringe as a 10% solution in Ultra Pure Elga water. The temperature of the tank was raised to 50°C and the reaction was allowed to proceed at 50°C. Ten minutes after the network had formed, the vial was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com