Method for producing antibodies from plasma cells
A technology of plasma cells and antibodies, applied in the field of producing antibodies from plasma cells, can solve problems such as expensive, unsuitable for high-throughput, and inefficient acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: Plasma cells in mesenchymal stromal cell cultures
[0095] The inventors observed primary cultures of human mesenchymal stromal cells established from normal bone marrow according to standard methods (Pittenger et al, 1999, Science 284:143-147; Bieback et al, 2004 Stem cells 22:625-634; Dominici et al, 2006, Cytotherapy 8:315-317; Sotiropoulou et al 2006, Stem Cells 24:462-471) contain antibody-secreting cells. These cells were detected by ELISPOT and identified as plasma cells. Plasma cells in mesenchymal stromal cell cultures were still detectable in vitro after 3 weeks (data not shown).
Embodiment 2
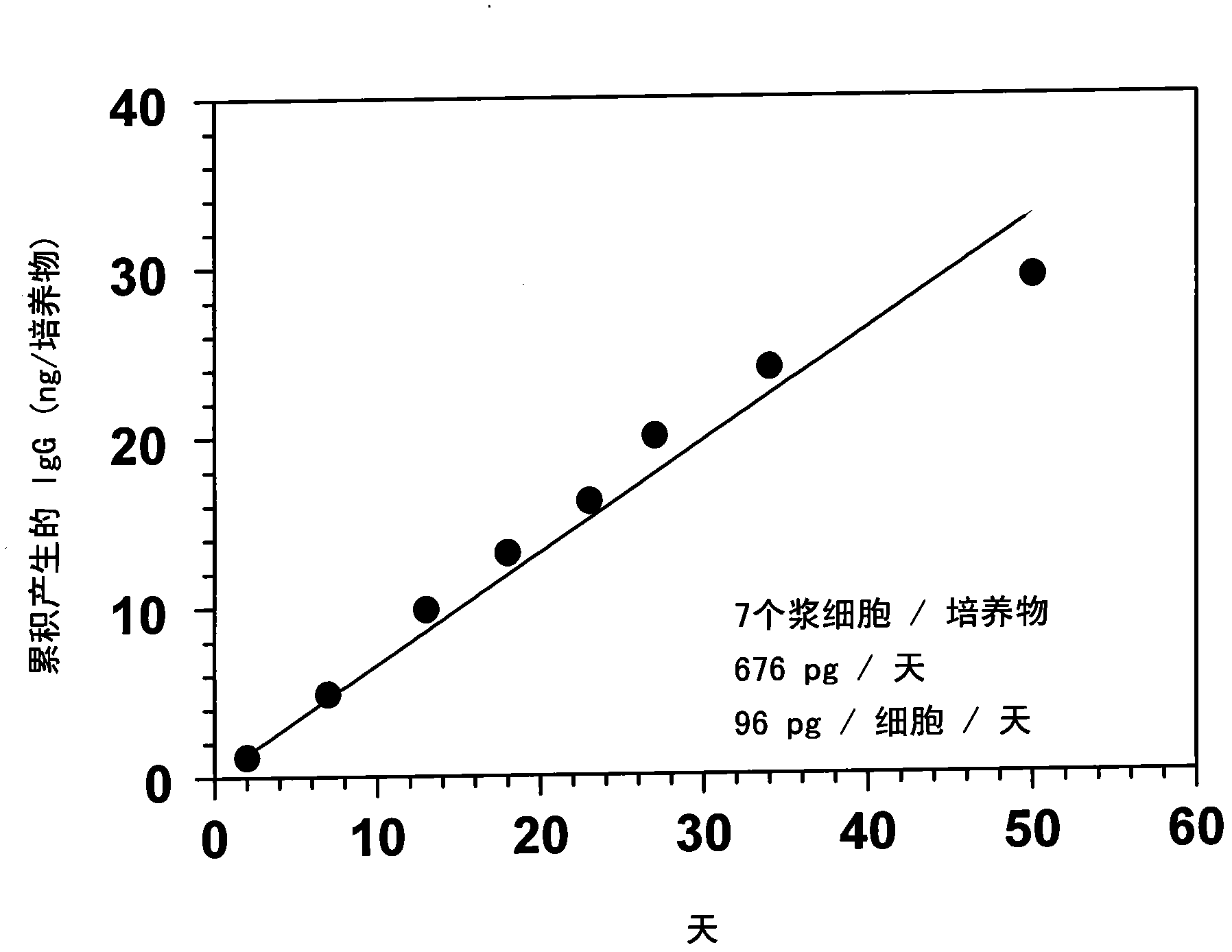
[0096] Example 2: Culture of human plasma cells up to 50 days
[0097] In order to develop a culture system in which individual plasma cells can remain viable so that the antibodies produced can accumulate over time in culture, the inventors tested different sources of primary mesenchymal stromal cells prepared according to standard methods. Briefly, tissue culture flasks were pre-coated with FCS for 1 hour. Make bone marrow cells supplemented with 30% FCS and 10 -8M dexamethasone was cultured overnight in complete IMDM medium. Unattached cells were washed away, and adherent cells were cultured in complete DMEM-10% FCS. Three of the seven lines tested supported human plasma cell survival but ceased proliferation after a few passages. In subsequent experiments, fixed mesenchymal stromal cells transduced with the telomerase reverse transcriptase gene (MSC-TERT) were used. These cells are those isolated by Mihara et al. (Br J Haematol 2003, 120, 846-849).
[0098] Peripheral...
Embodiment 3
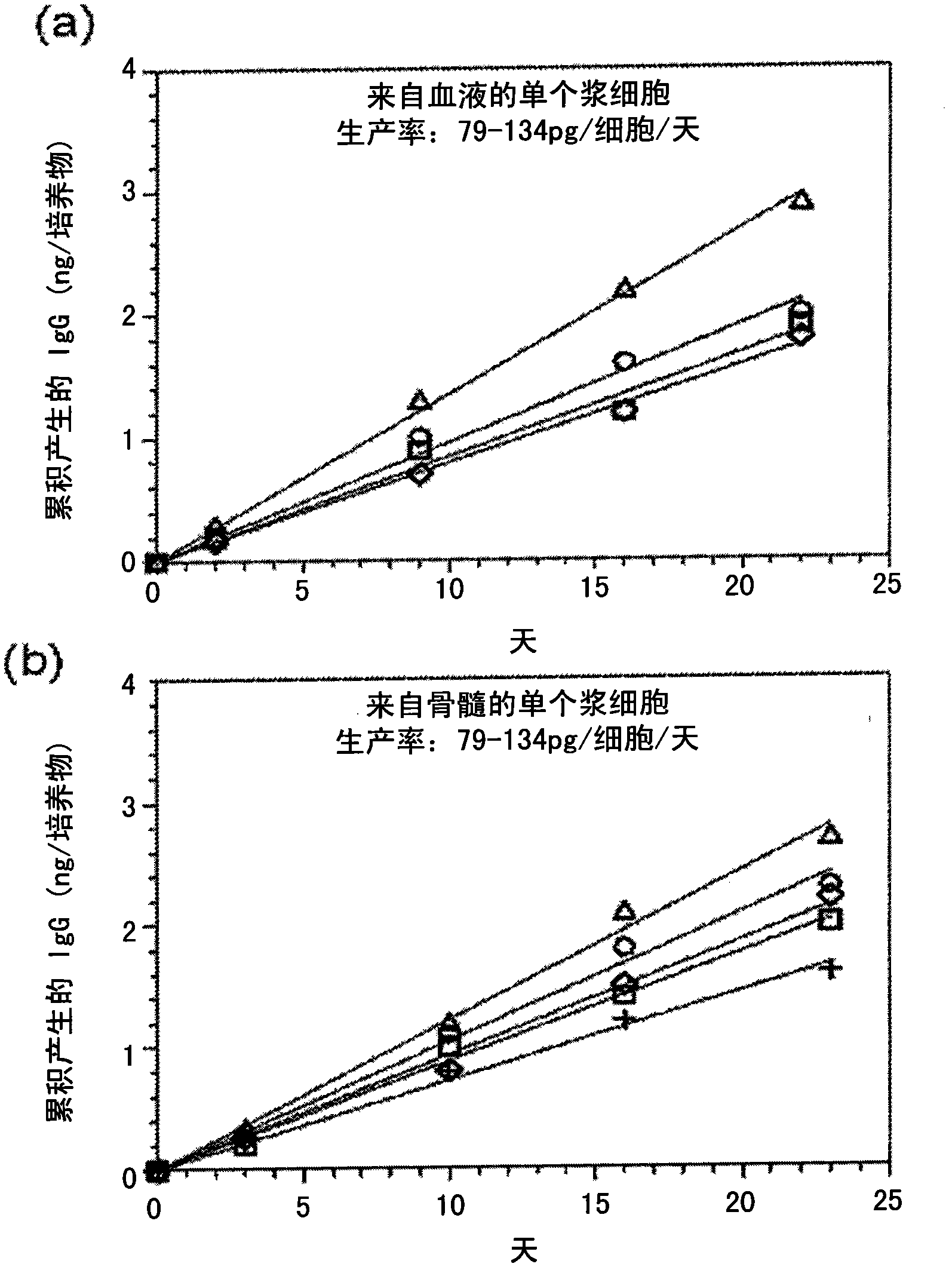
[0099] Example 3: 3-week cultures of individual plasma cells
[0100] Isolation of plasma cells from peripheral blood or bone marrow using PE-conjugated anti-CD138 antibody followed by anti-PE microbeads and cell sorting, and seeding mesenchymal matrix in 96-well plates at a density of 0.5 cells / well on a cell monolayer. IgG-containing cultures were monitored over a 22-23 day period by taking regular samples. Medium was changed on day 16. IgG productivity in monoclonal cultures was constant at 72-134 pg / cell / day throughout the culture period ( figure 2 a, Peripheral blood-derived (4 cultures); figure 2 b, Bone marrow derived (5 cultures)).
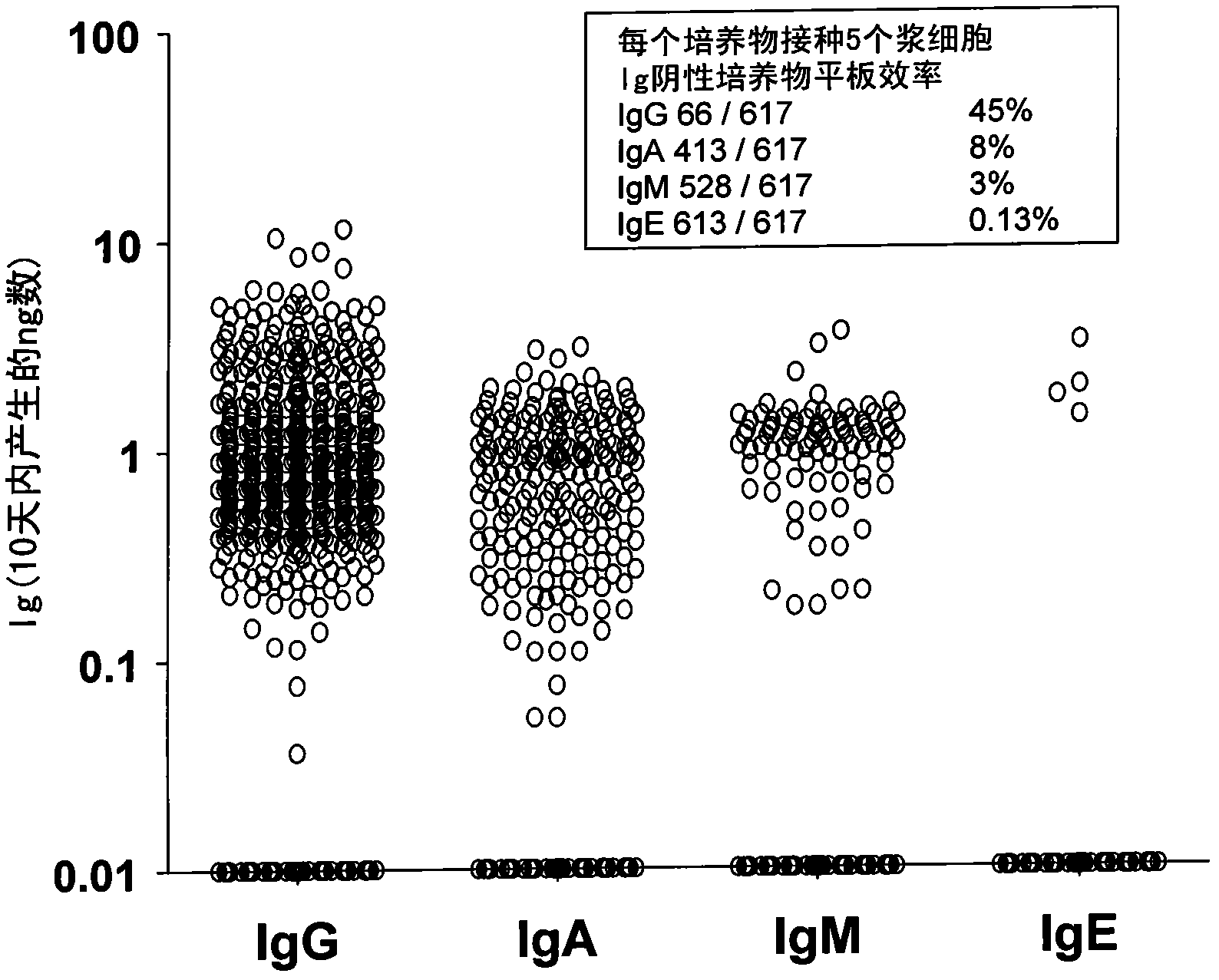
[0101] Plating efficiencies of blood and bone marrow plasma cells ranged from 30% to 65% in 5 limiting dilution experiments (data not shown). In addition, plasma cells recovered from polyclonal cultures can be replated into unicellular cultures where they maintain a constant rate of Ig secretion (data not shown). The linear accumul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com