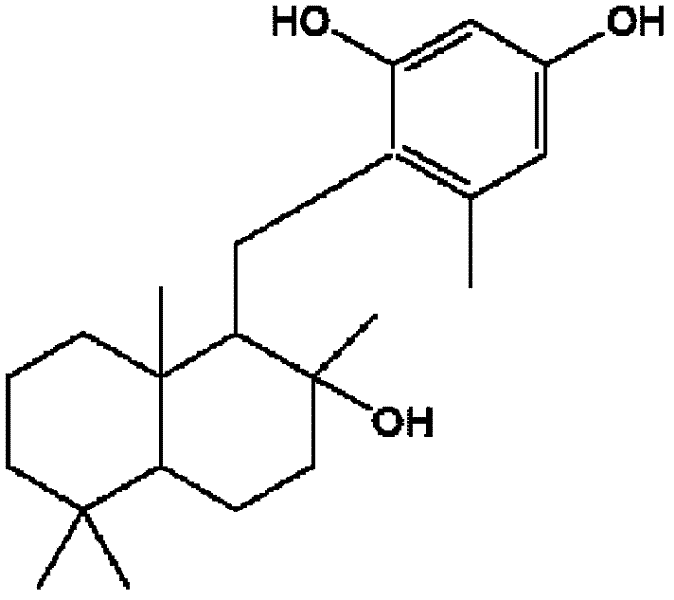
A kind of natural small molecular compound and its application in the preparation of antitumor drugs
A technology of compounds and extracts, which is applied in the field of natural small molecule compounds, can solve the problems of cell tumor growth and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1. Isolation and identification of Neoalbaconol.
[0051] Collect complete and fresh fruiting bodies of Albatrellus confluens and soak them in 95% ethanol. After standing at room temperature for 7 days, take the ethanol solution and evaporate to dryness to obtain ethanol extract; weigh the extract residue (0.715kg) and Pulverize, soak for one day at room temperature through dichloromethane / methanol (volume ratio 1: 1), get dichloromethane / methanol solution and evaporate to dryness, obtain extract, repeat extraction like this 6 times; Merge the extract that each extraction obtains, use Chloroform / ethyl acetate mixed solvent (volume ratio 8: 2) was dissolved and separated by 50mm×100cm silica gel column chromatography, the eluent was chloroform / ethyl acetate mixture (volume ratio 8: 2), and the elution speed It is 1 ml per minute; from the first minute, the eluent collected by the chromatography column every 10 minutes is a fraction, wherein fractions 1-4 are evap...
Embodiment 2
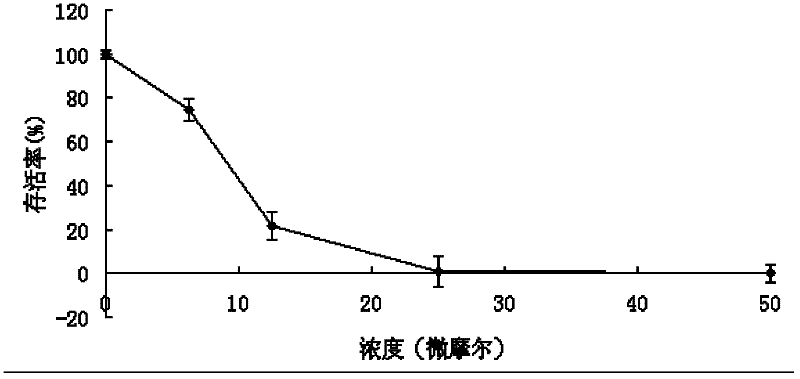
[0059] Example 2. Neoalbaconol inhibits the proliferation of various tumor cell lines.
[0060] The inhibitory effect of the compound Neoalbaconol on tumor cell proliferation was observed by Am-Blue method, and the 50% inhibitory concentration (IC50 value) of its proliferation activity was determined. Am-Blue (SUNBIO Company) is a redox indicator used for detection of cell proliferation and toxicity. It is purple-blue and non-fluorescent in the oxidized state, and turns into a pink or red fluorescent reduction product in the reduced state. Its absorption peak is It is 530-560nm, and the scattering peak is 590nm.
[0061] During the process of cell proliferation, the ratios of NADPH / NADP, FADH / FAD, FMNH / FMN and NADH / NAD in cells increase, and they are in a reducing environment. After the Am-Blue reagent is taken into the cell, the oxidized blue indicator is reduced by the mitochondria of the cell and released outside the cell, making the medium turn fluorescent pink or red. U...
Embodiment 3
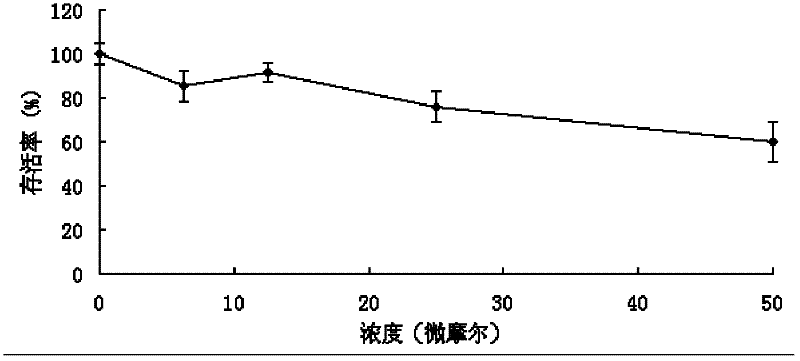
[0070] Example 3. Neoalbaconol induces apoptosis in tumor cell lines.
[0071] In order to observe the apoptosis-inducing effect of the compound Neoalbaconol on tumor cells, the C666-1 nasopharyngeal carcinoma cell line was treated with different concentrations of the compound, the nuclei were stained with ethidium bromide (PI), and the stained cells were detected by flow cytometry. Ratio, from which the ratio of late apoptotic cells can be judged.
[0072] The operation steps are as follows: the C666-1 cell line was treated with 0 μM, 5 μM, 10 μM, and 20 μM Neoalbaconol for 24 hours and 48 hours respectively; the treated cells were collected by trypsinization without EDTA, and the cells were washed twice with PBS (centrifugation 2000rpm, 5min) to collect 1~5×10 5 Cells were fixed in pre-cooled 70% ethanol; 5 μL PI was added and mixed; protected from light and reacted at room temperature for 5 minutes; flow cytometry (Ex=488nm; Em=530nm) was used to detect PI-positive cells. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com