Preparation method of (2r,3r,11br)-dihydrotetrabenazine and related compounds
A technology of dihydrotetrabenazine and tetrabenazine, which is applied in the direction of organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as difficult industrial production, avoid column chromatography purification process, and improve three-dimensional Effects of Selectivity and Chemical Yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
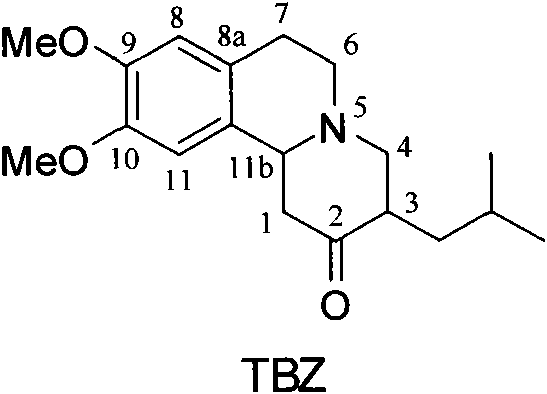
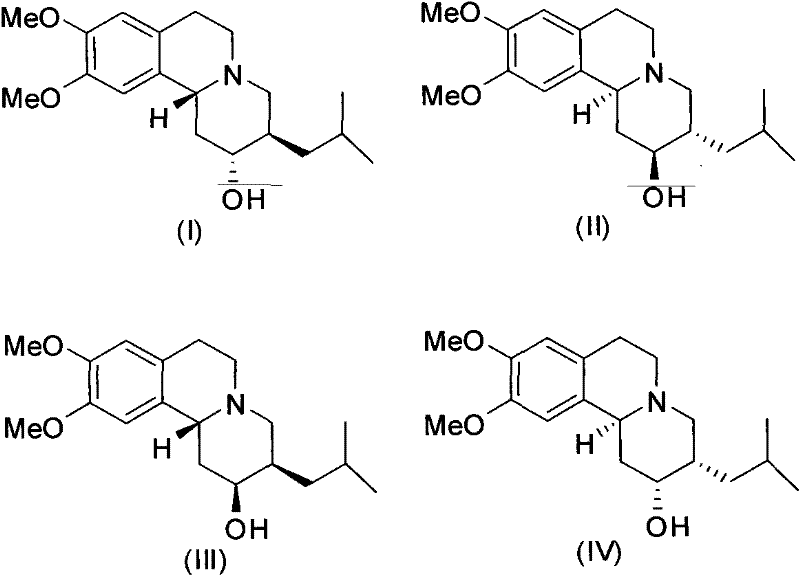
[0022] (2R, 3R, 11bR)-dihydrotetrabenazine ((2R, 3R, 11bR)-DHTBZ)
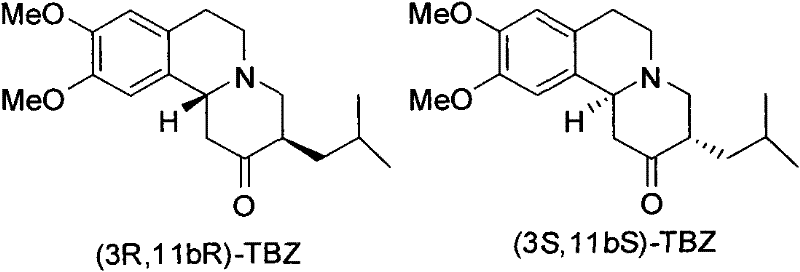
[0023] (3R, 11bR)-TBZ (1.0g, 3.2mmol) was dissolved in 11ml of tetrahydrofuran, and after the solution was cooled to -20°C, 2M borane-dimethylsulfide-tetrahydrofuran (3.2ml, 6.4mmol) was added dropwise into the reaction solution. After the reaction solution was stirred and reacted at -20°C for 2 h, 11 ml of ammonia water was added, and then the mixed solution was heated to 35°C and stirred overnight. Saturated brine was added, and extracted with ether. The ether extract was washed with saturated NaCl solution, anhydrous Na 2 SO 4 It was dried, filtered, and the solvent was distilled off under reduced pressure to obtain 1.07 g of a white solid. The crude product was recrystallized twice through acetone-water to obtain the pure product (2R, 3R, 11bR)-dihydrotetrabenazine (0.64g white solid, yield 64%), [α] D 21 =+58.93° (c 0.6, MeOH). 1 H NMR (300MHz, CDCl 3 ): δ6.68(s, 1H), 6.58(s, 1H), 3.84(s, 6H), 3.3...
Embodiment 2
[0025] (2S,3S,11bS)-dihydrotetrabenazine ((2S,3S,11bS)-DHTBZ)
[0026] According to the experimental procedure described in Example 1, (3S, 11bS)-TBZ was reduced with borane-dimethyl sulfide to obtain (2S, 3S, 11bS)-dihydrotetrabenazine (white solid, yield 63%) , [α] D 22 =-56.1° (c 0.23, MeOH). 1H NMR (300MHz, CDCl 3 ): δ6.67(s, 1H), 6.58(s, 1H), 3.87(s, 6H), 3.43-3.34(m, 1H), 3.14-2.96(m, 4H), 2.65-2.54(m, 2H) ), 2.53 (ddd, 1H, J=11.1, 11.1, 3.6Hz), 2.01-1.94 (m, 1H), 1.77-1.64 (m, 2H), 1.62-1.36 (m, 2H), 1.10-0.99 (m , 1H), 0.95-0.70 (m, 6H); 13C NMR (75MHz, CDCl 3 )δ147.50, 147.23, 129.36, 126.42, 111.49, 107.96, 74.63, 60.88, 60.08, 55.94, 55.84, 51.89, 41.65, 40.58, 39.70, 29.18, 25.35, 24.12, 32.76; ESI-MS m / z: M+H]+, HRMS-EIS (m / z): 320.2242 [M+H]+ (Calcd for C19H30NO3 320.2226).
Embodiment 3
[0028] (±)-α-dihydrotetrabenazine ((±)-α-DHTBZ)
[0029] According to the experimental procedure described in Example 1, tetrabenazine (TBZ) was reduced with borane-dimethyl sulfide to obtain (±)-α-dihydrotetrabenazine (white solid), 1 H NMR (300MHz, CDCl 3 )δ6.68(s, 1H), 6.58(s, 1H), 3.84(s, 6H), 3.39(m, 1H), 3.16-2.97(m, 4H), 2.67-2.41(m, 3H), 1.99 (t, J=11.3Hz, 1H), 1.75-1.44 (m, 5H), 1.10-1.01 (m, 1H), 0.93 (t, J=6.5Hz, 6H); ESI-MS m / z 320.3 [M +H] + , 342.3[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com