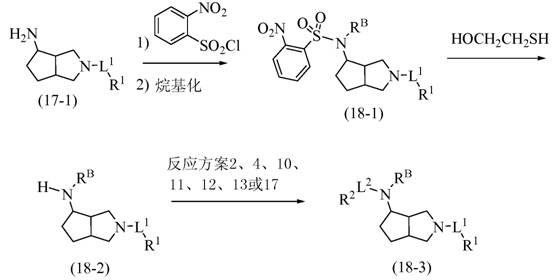
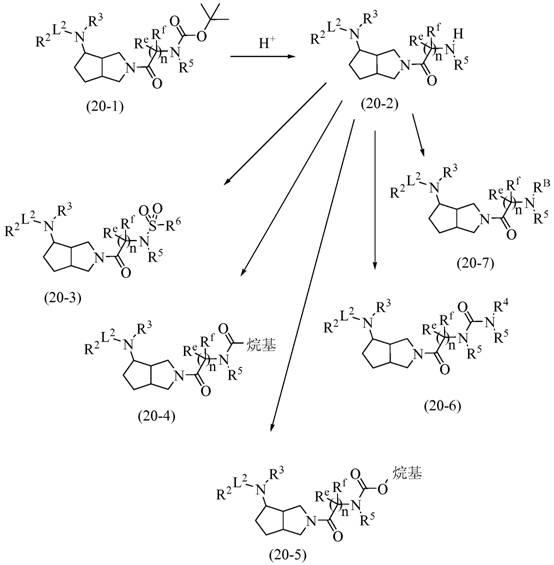
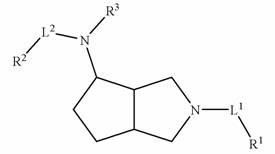
Substituted octahydrocyclopentadieno(c)pyrrol-4-amines as calcium channel blockers
A technology of octahydrocyclopentadiene and trityl octahydrocyclopentadiene, applied in the field of substituted octahydrocyclopentadiene (C) pyrrol-4-amine as a calcium channel blocker, capable of Solve the problem of no effect, no effect of thermal hyperalgesia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and Embodiment 2
[0830] N -[(3a S *, 4 S *, 6a R *)-2-benzyl octahydrocyclopenta[ c ]Pyrrol-4-yl]-1-phenylcyclopentanecarboxamide (Example 1) and
[0831] N -[(3a S *, 4 R *, 6a R *)-2-benzyl octahydrocyclopenta[ c ]Pyrrol-4-yl]-1-phenylcyclopentanecarboxamide (Example 2)
[0832] Combine 1-hydroxybenzotriazole (33 mg, 0.24 mmol) and N -(3-Dimethylaminopropyl)- N '-Ethylcarbodiimide (43 μL, 0.24 mmol) was added to 1-phenylcyclopentanecarboxylic acid (46 mg, 0.24 mmol) in dichloromethane (2 mL). Stir the reaction at room temperature for 10 minutes, then add (3a S *, 6a R *)-2-benzyl octahydrocyclopenta[ c ]Pyrrol-4-amine (52 mg, 0.24 mmol) and the reaction was stirred overnight at room temperature. The reaction was terminated with water and extracted with dichloromethane, and then chromatographed on silica gel with 1-10% methanol (2 N Ammonia / chloroform was used as the eluent to obtain the title compound.
Embodiment 1
[0833] Example 1: 1 H NMR (500 MHz, pyridine -d 5 ) δ ppm 7.52-7.28 (m, 9H), 7.25 (d, J = 7.7, 1H), 4.43-4.34 (m, 1H), 3.56 (d, J = 12.9, 1H), 3.17 (d, J = 12.9, 1H), 2.83-2.67 (m, 2H), 2.59-2.50 (m, 1H), 2.50-2.44 (m, 1H), 2.35-2.29 (m, 2H), 2.04-1.87 (m, 4H) , 1.84-1.54 (m, 6H), 1.43-1.26 (m, 1H), 1.04-0.94 (m, 1H); MS (ESI+) m / z 389 (M+H) + .
Embodiment 2
[0834] Example 2: 1 H NMR (500 MHz, pyridine -d 5 ) δ ppm 7.52-7.47 (m, 2H), 7.41 (d, J = 7.4, 2H), 7.38-7.29 (m, 4H), 7.25 (q, J = 7.3, 2H), 4.36 (m, 1H), 3.57 (d, J = 13.2, 1H), 3.40 (d, J = 13.2, 1H), 2.78 (m, 3H), 2.33 (m, 3H), 2.27 (d, J = 8.7, 1H), 2.21-2.13 (m, 1H), 1.99 (dt, J = 5.8, 11.7, 3H), 1.87-1.75 (m, 2H), 1.71-1.60 (m, 3H), 1.44 (ddd, J = 7.7, 12.1, 14.5, 1H), 1.31 (m, 1H); MS (ESI+) m / z 389 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com