Polymorphs of darunavir
A technology of darunavir and crystallization, which is applied in the field of application in the treatment of retrovirus infection, can solve unmet problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: Preparation of crystalline tetrahydrofuran solvate of darunavir
[0087] The darunavir tetrahydrofuran solvate of the present invention is prepared by dissolving approximately 1 g of darunavir ethanolate in 5 ml of tetrahydrofuran solvent. The solvent was then evaporated at room temperature (approximately 25°C) until crystals formed.
[0088] Alternatively, the darunavir tetrahydrofuran solvate of the invention is prepared by dissolving darunavir ethanolate in a tetrahydrofuran solvent, followed by addition of the antisolvent isopropanol (IPA) to cause crystal precipitation.
[0089] Alternatively, darunavir ethanolate was dissolved in a 1:2 ratio of tetrahydrofuran (THF):isopropyl acetate (iPrOAc) or a 1:2 ratio of tetrahydrofuran (THF):methyl tert-butyl ether ( MTBE) the darunavir tetrahydrofuran solvate of the present invention was prepared by heating the mixture to 60°C followed by cooling using an ice bath to induce crystallization.
Embodiment 2
[0090] Example 2: Characterization of the crystalline tetrahydrofuran solvate of darunavir
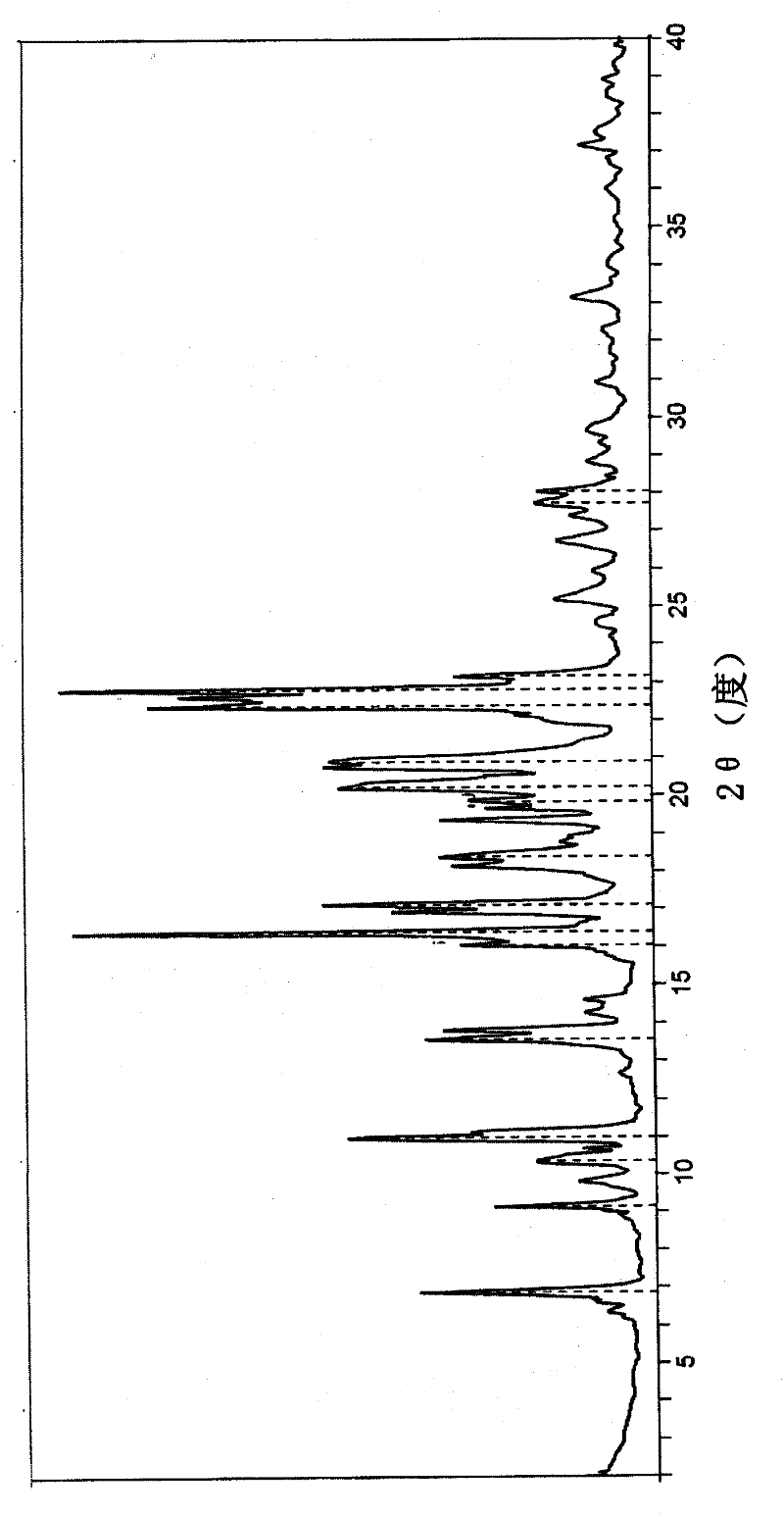
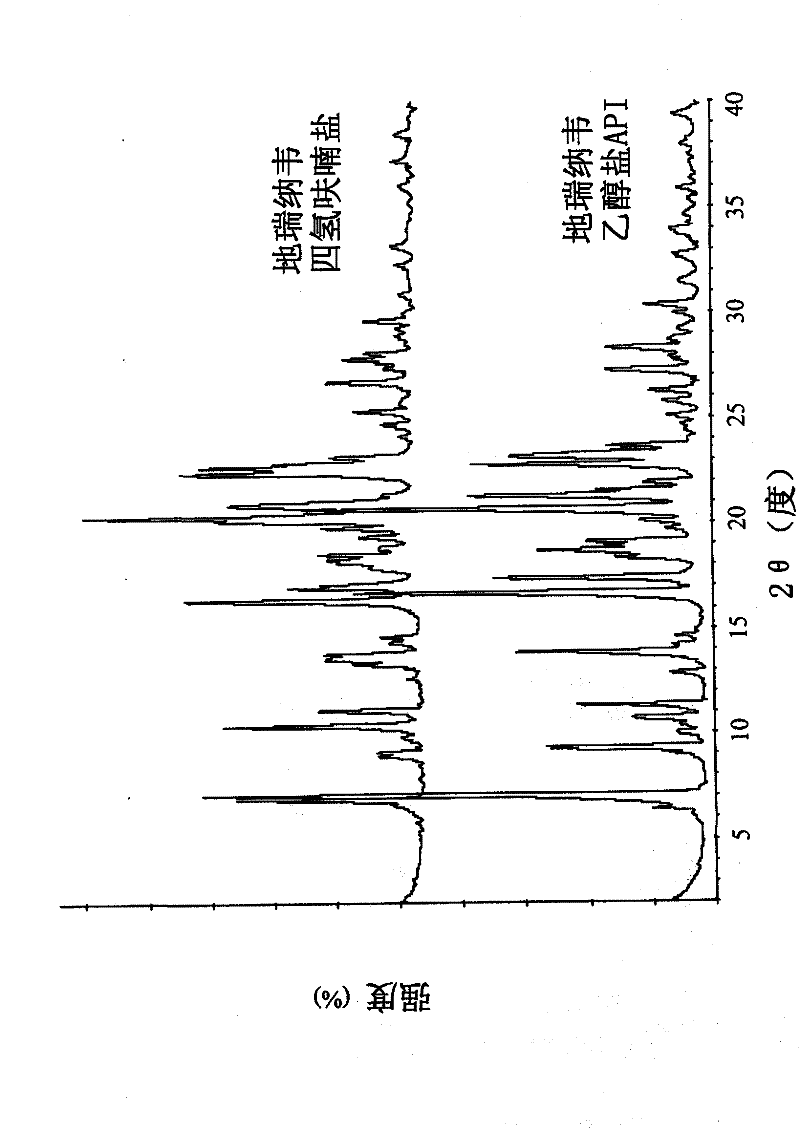
[0091] This new polymorph exhibits an endothermic peak at -95°C in differential scanning calorimetry (DSC; Mettler Toledo DSC; 10°C / min). X-ray powder diffraction (XRPD; Rigaku D / MAX 2200, CuKα, 40kV, 40mA, DivSlit 1deg, DivH.L.Slit 10mm, SctSlit 1deg, RecSlit 0.3mm, 10deg / min) showed unique characteristic peaks ( figure 1 ;Table 1). The X-ray powder diffraction pattern of the tetrahydrofuran solvate of darunavir of the present invention has a unique fingerprint, which is different from the X-ray diffraction pattern of darunavir ethanolate API ( figure 2 ). Even after two weeks of storage at 25°C, the XRPD and DSC spectra remained unchanged, thus indicating the stability of the crystals.
[0092] Table 1: X-ray diffraction peaks of darunavir tetrahydrofuran solvate
[0093]
[0094]
[0095] * Relative intensities may vary between samples.
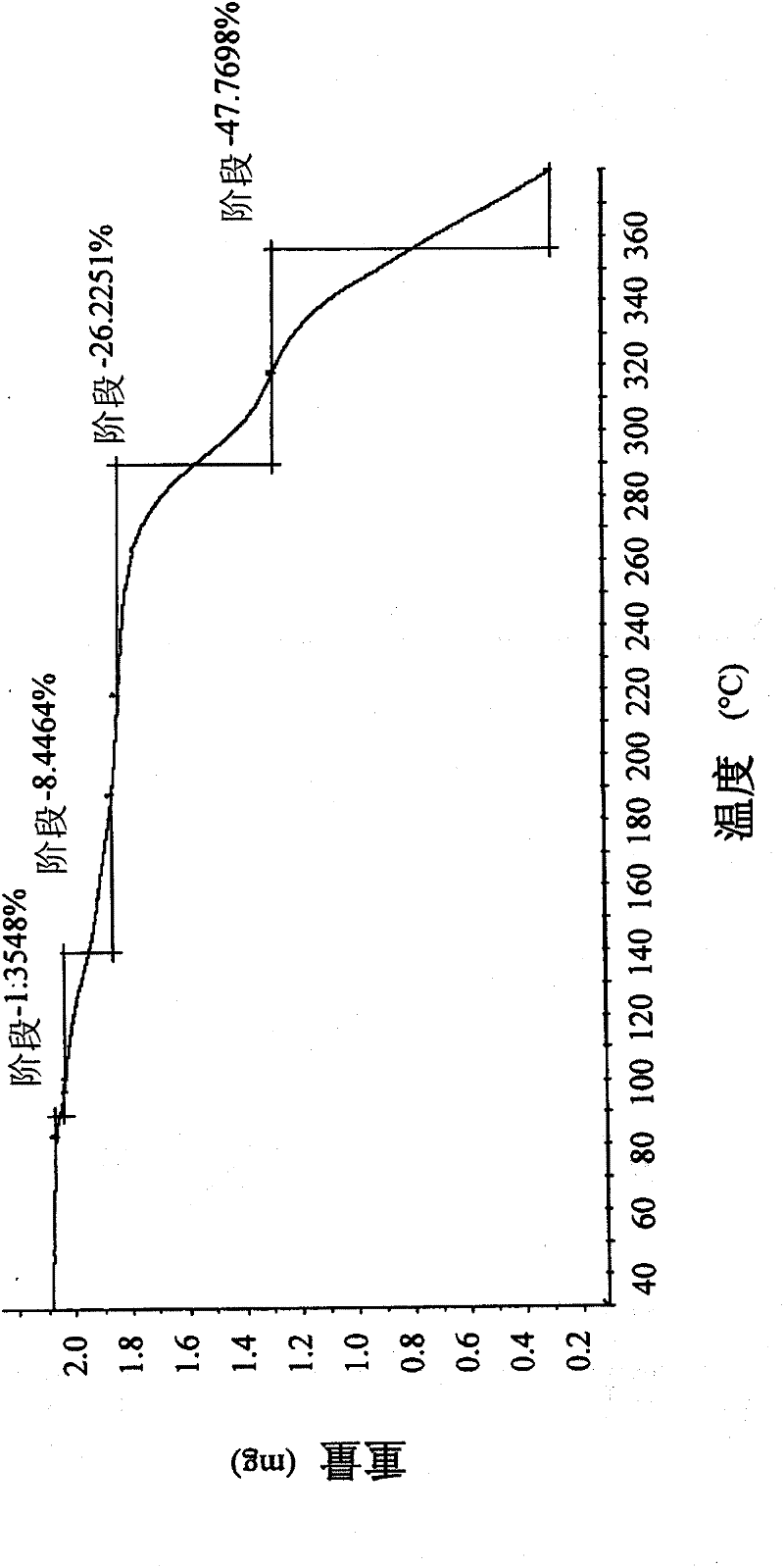
[0096] Thermogravimetric anal...
Embodiment 3
[0109] Example 3: Preparation of crystalline dimethyl sulfoxide solvate of darunavir
[0110] The darunavir dimethyl sulfoxide solvate of the present invention was prepared by dissolving darunavir ethoxide in dimethyl sulfoxide at 60°C, followed by cooling using an ice bath to induce crystallization.
[0111] Alternatively, the darunavir dimethyl sulfoxide solvate of the present invention is prepared by dissolving approximately 1 g of darunavir ethanolate in 2.5 ml of dimethyl sulfoxide at 80°C. Water (10ml) was then added to induce crystallization.
[0112] Alternatively, the darunavir dimethyl sulfoxide solvate of the invention was prepared by dissolving darunavir ethoxide in dimethyl sulfoxide, followed by addition of the antisolvent isopropanol (IPA) to cause crystal precipitation.
[0113] Alternatively, the darunavir dimethyl sulfoxide solvate of the present invention is prepared by dissolving darunavir ethanolate at 60°C in any one of the following solvent mixtures: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com