Pyrimidinecarboxamide derivatives as inhibitors of SYK kinase
A technology of drugs and compounds, applied in the direction of anti-inflammatory agents, drug combinations, non-central analgesics, etc., can solve the problem of lack of efficacy of placebos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
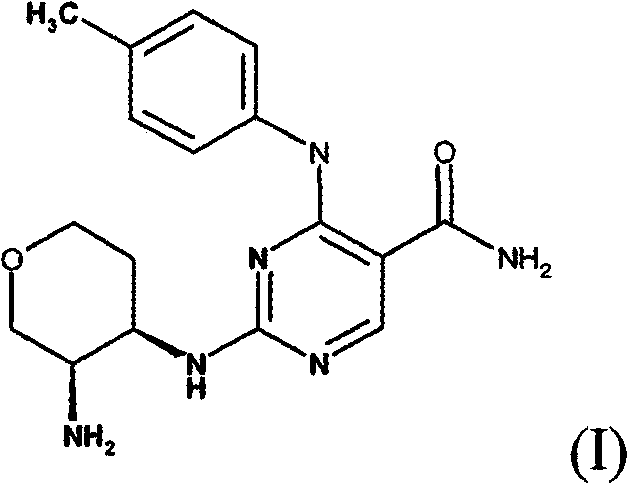
[0301] Example 1-2-{[(3R, 4R)-3-aminotetrahydro-2H-pyran-4-yl]amino}-4-[(4-methylphenyl)amino]-5-pyrimidinemethyl Amide
[0302]
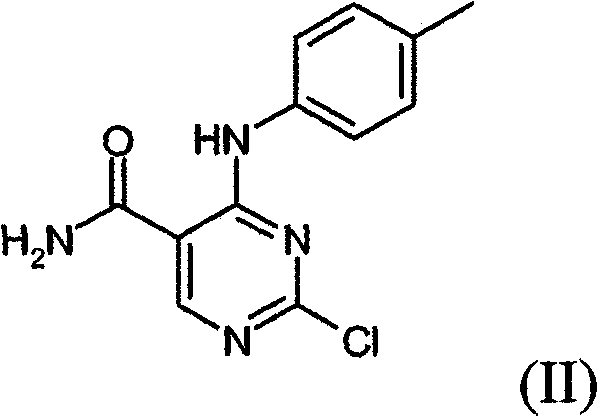
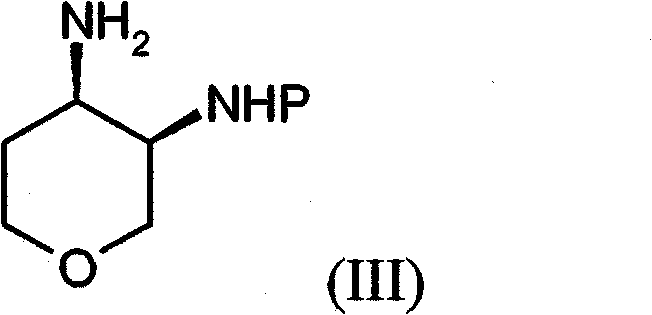
[0303] [(3R, 4R)-4-({5-(aminocarbonyl)-4-[(4-methylphenyl)amino]-2-pyrimidinyl}amino)tetrahydro-2H-pyran-3- 1,1-Dimethylethyl carbamate (52.2g) was added to a mixture of hydrogen chloride in isopropanol (5M, 300ml) and ethanol (400ml). The mixture was heated to reflux with stirring and heating was continued for 24 hours with vigorous stirring. The mixture was cooled to room temperature, filtered and the solid was washed with ethanol (100ml) and dried in vacuo. The crude product was suspended in water (900ml) and heated to reflux to give a clear solution. The solution was basified with sodium hydroxide solution (2M, 300ml) and cooled in ice. The precipitated product was collected by filtration, washed with water (2×100 ml) and the beige solid was dried in a vacuum oven at 40° C. for 2 h to give 2-{[(3R,4R)-3-aminotetrahydro- 2H-pyran-4-yl]am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com