Novel vaccine adjuvant and application
A new type of vaccine adjuvant and vaccine adjuvant technology, which is applied in the field of SPO1 adjuvant, oil-in-water vaccine adjuvant and its preparation, achieves the effect of low cost, suitable for mass production industrialization, and avoiding cross-infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1: the preparation of novel vaccine adjuvant
[0098] Squalene 5.0g
[0099] Polyoxyethylene castor oil 0.3g
[0100] Polyether 0.3g
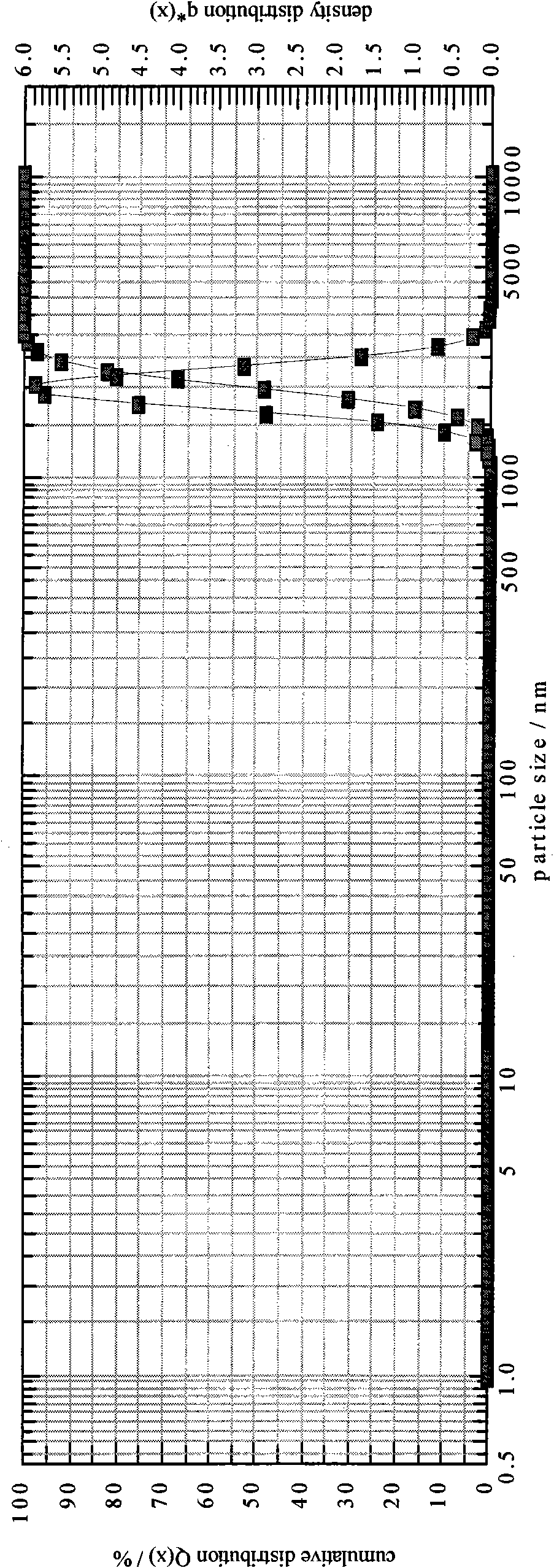
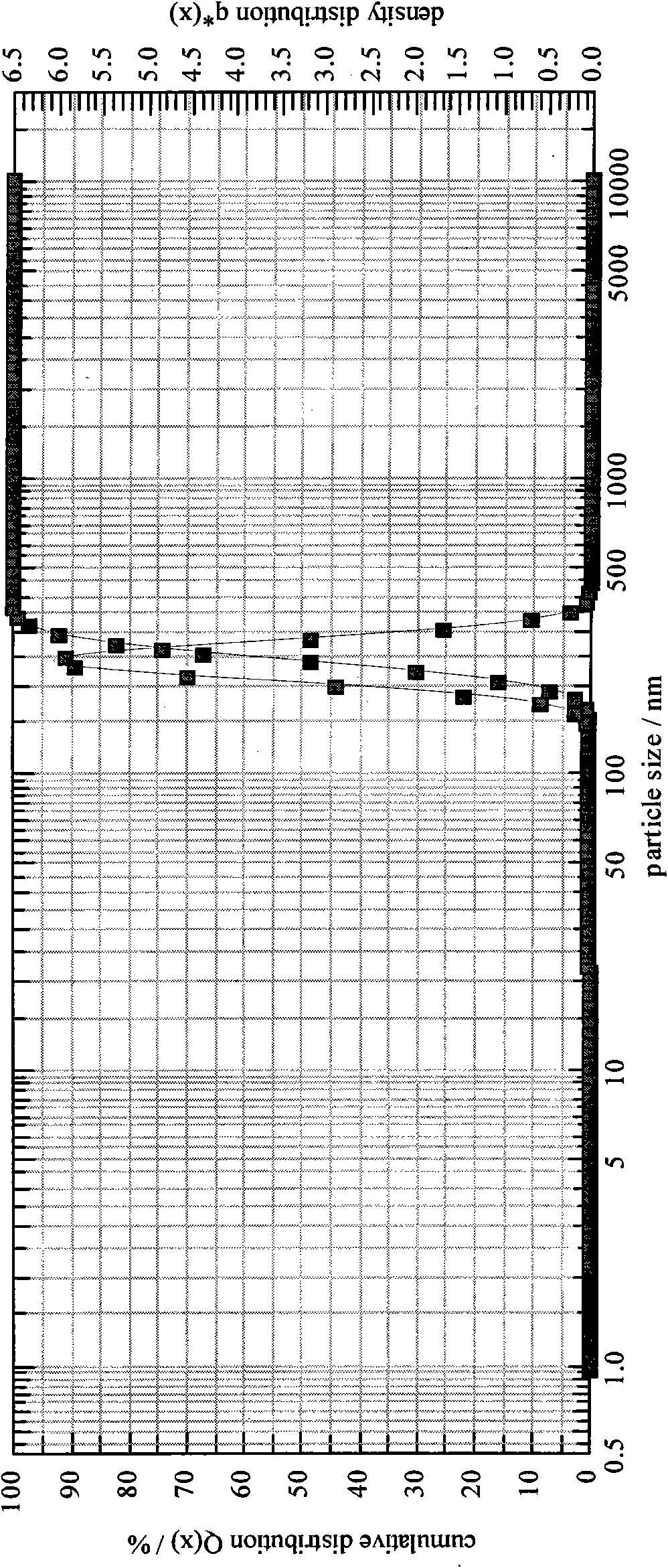
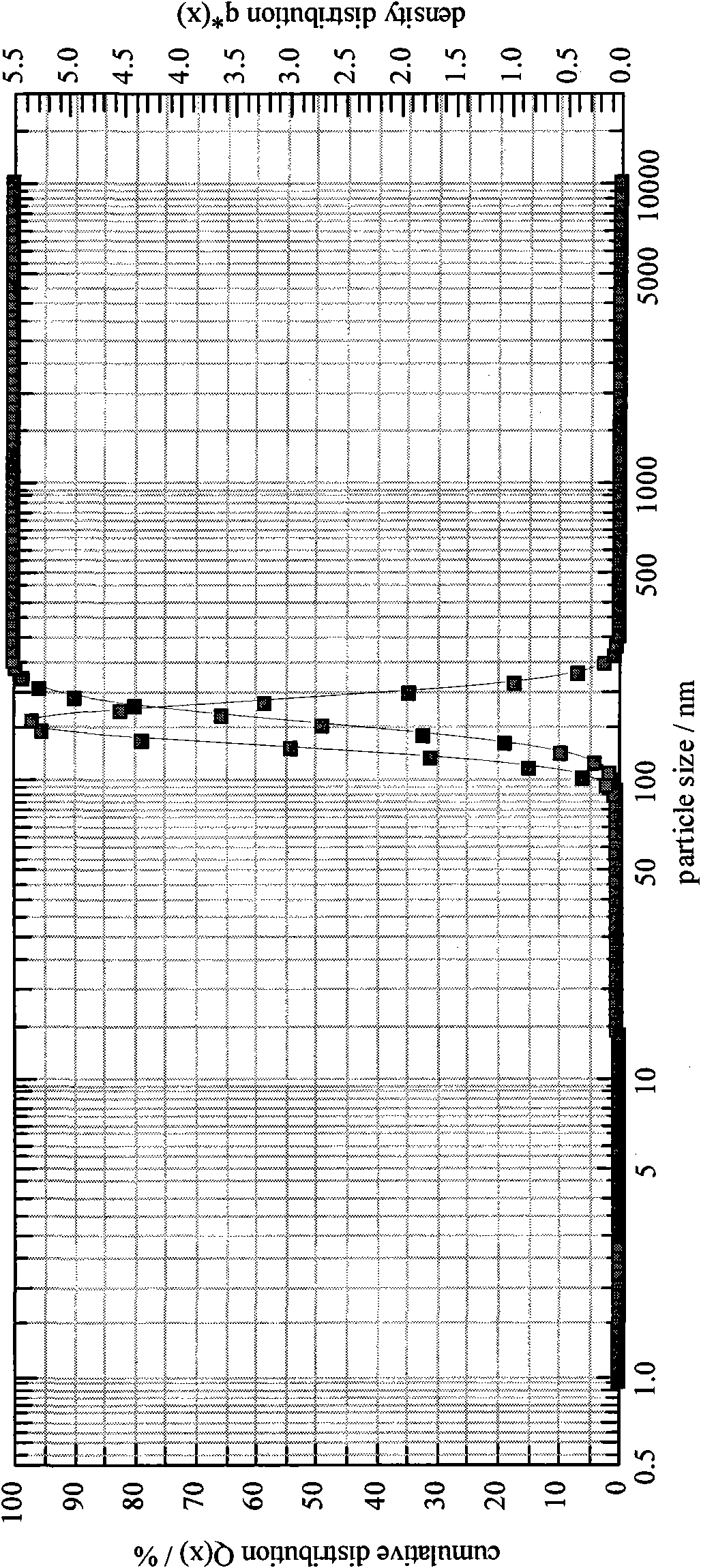
[0101] Take the raw materials and use a homogenizer to prepare colostrum. The homogenization conditions are: rotating speed 6000rpm, time 4 minutes; the particle size of the colostrum is x 10 =1612.39nm,x 50 =1969.24nm,x 90 =2401.49nm, SMD=1946.88nm, VMD=1992.92nm; homogeneous colostrum: the homogeneous condition is a pressure of 5000psi, 6 cycles, and the particle size is measured as x 10 =197.10nm,x 50 =240.37nm,x 90=292.69nm, SMD=237.53nm, VMD=243.16nm; homogeneous again, homogeneous condition: pressure 10000psi, 7 cycles, measure its particle size as x 10 =125.12nm,x 50 =155.54nm,x 90 = 193.14nm, SMD = 153.30nm, VMD = 157.78nm; after preparing a milky white vaccine adjuvant preparation, add deionized water as buffer to 100ml, filter and sterilize with a 0.22μm filter to obtain the product.
Embodiment 2
[0102] Embodiment 2: the preparation of novel vaccine adjuvant
[0103] Squalene 7.0g
[0104] Polyoxyethylene castor oil 2.0g
[0105] Polyether 1.5g
[0106] Take the raw materials and use a homogenizer to prepare colostrum. The homogenization conditions are: ultrasonic to prepare colostrum, ultrasonic 7 times, 45s / time, the particle size of colostrum is x 10 =1623.75nm,x 50 =1958.31nm,x 90 =2400.35nm, SMD=1948.22nm, VMD=1989.54nm; homogeneous colostrum: homogeneous condition is pressure 6000psi, 7 cycles, measure its particle size as x 10 =197.10nm,x 50 =240.37nm,x 90 =292.69nm, SMD=237.53nm, VMD=243.16nm; homogeneous again, homogeneous condition: pressure 12000psi, 7 cycles, measure its particle size as x 10 =126.13nm,x 50 =156.58nm,x 90 = 194.15nm, SMD = 154.36nm, VMD = 158.79nm, after preparing milky white vaccine adjuvant preparations, add physiological saline as the buffer to 100ml, filter and sterilize with a 0.22μm filter to obtain the final product.
Embodiment 3
[0107] Embodiment 3: the preparation of novel vaccine adjuvant
[0108] Squalene 3.0g
[0109] Polyoxyethylene castor oil 4.0g
[0110] Polyether 3.5g
[0111] Take the raw materials and use a homogenizer to prepare colostrum. The homogenization conditions are: rotating speed 8000rpm, time 3min; the particle size of colostrum is x 10 =1620.79nm,x 50 =1968.33nm,x 90 =2410.65nm, SMD=1950.12nm, VMD=1987.51nm; homogeneous colostrum: the homogeneous condition is a pressure of 7000psi, 5 cycles, and the particle size is measured as x 10 =193.10nm,x 50 =238.55nm,x 90 =290.66nm, SMD=234.53nm, VMD=240.36nm; homogeneous again, homogeneous condition: pressure 14000psi, 6 cycles, measure its particle size as x 10 =123.82nm,x 50 =150.54nm,x 90 = 190.74nm, SMD = 149.30nm, VMD = 157.78nm, after preparing milky white vaccine adjuvant preparation, select phosphate buffer solution to add to 100ml, filter and sterilize with 0.22μm filter to get final product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com