Production method for spirodiclofen
A production method and a technology for spirodiclofen, applied in the field of production technology of spirodiclofen, can solve the problems of low reaction yield and high production cost, and achieve the effects of easy availability of raw materials, easy operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
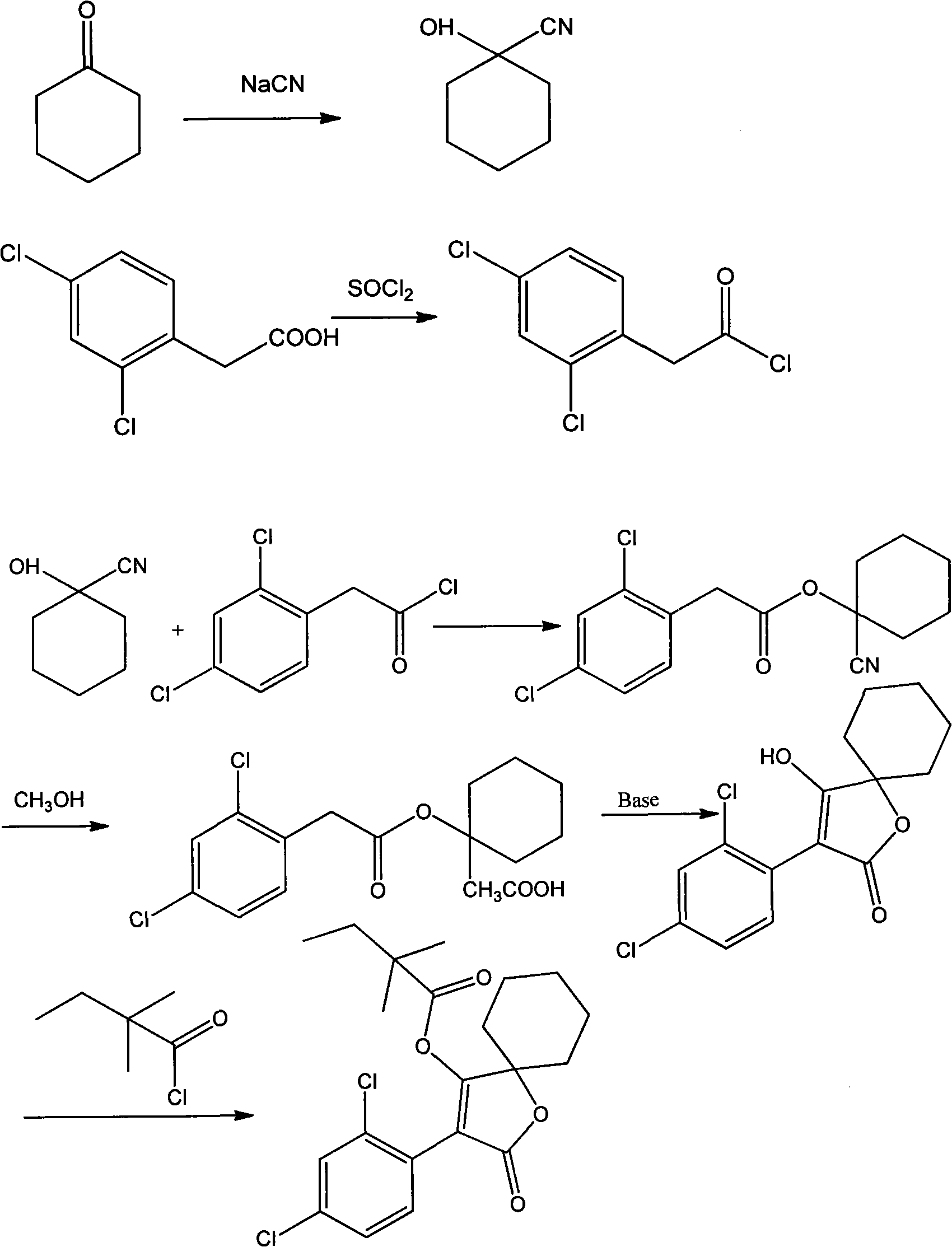
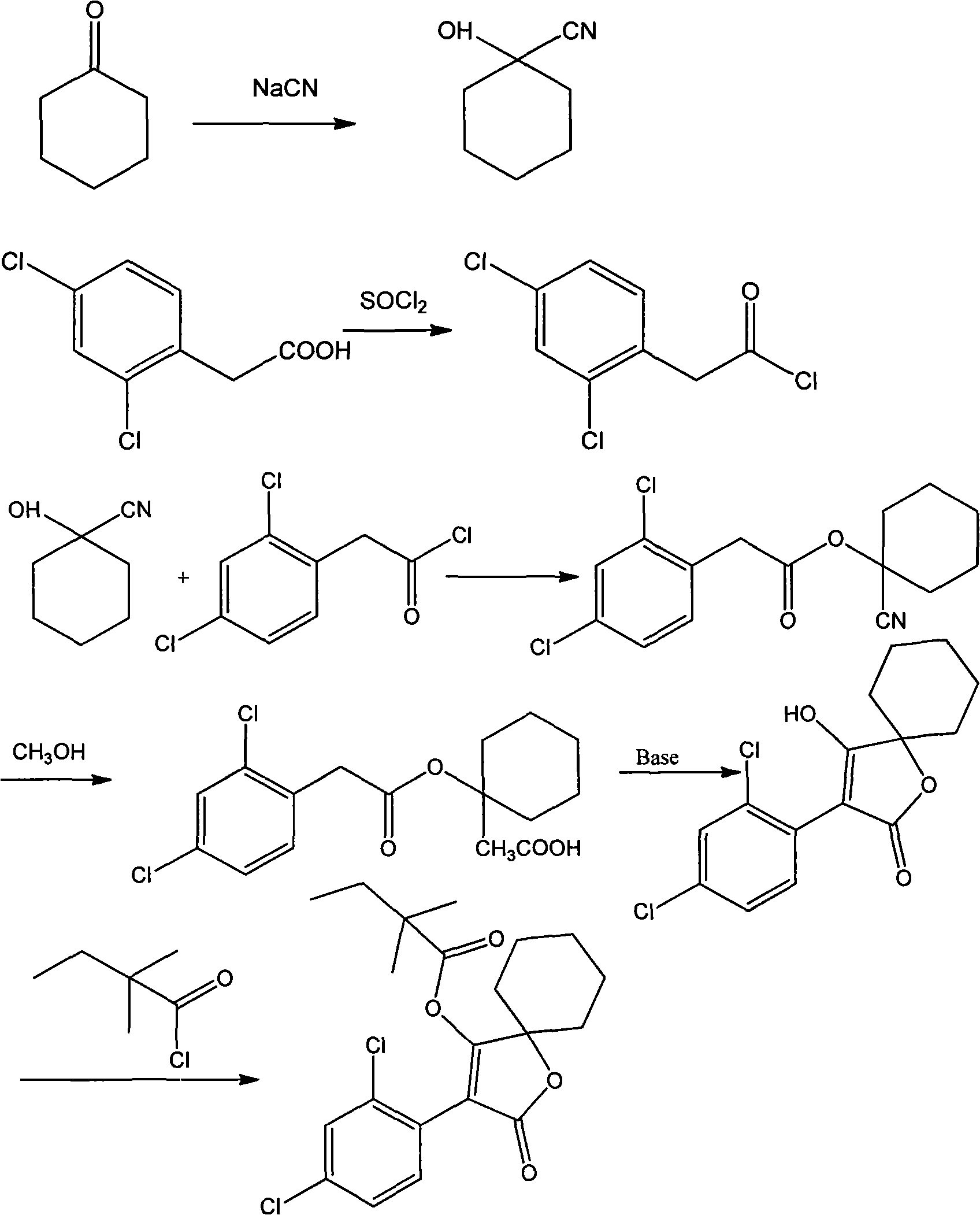
[0010] Preparation of 1-cyanocyclohexanol
[0011] Under mechanical stirring, add 60.9g cyclohexanone, 24.1g sodium cyanide, 80ml water in a 500ml three-necked flask, keep the temperature at 5-10℃ under ice bath, slowly add 40% sulfuric acid solution dropwise, keep dropping The addition time is 3h. After the addition is completed, stirring is continued for 30min. The product is extracted with ethyl acetate. The combined ethyl acetate layer is washed with water, dried with anhydrous sodium sulfate, and concentrated to obtain 66.8g of liquid product with a yield of 86.0%. Purified for the next reaction.
[0012] Preparation of 2,4-Dichlorophenylacetyl chloride
[0013] Add 2.6g of 2,4-dichlorophenylacetic acid and 30ml of thionyl chloride into the reaction flask, heat at 75-100℃, reflux and stir the reaction for 4h, distill under reduced pressure to remove thionyl chloride to obtain 2.80g of yellow liquid, yield 98.8 %.
[0014] Preparation of 2,4-Dichlorophenylacetic acid-1-cyanocycl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com