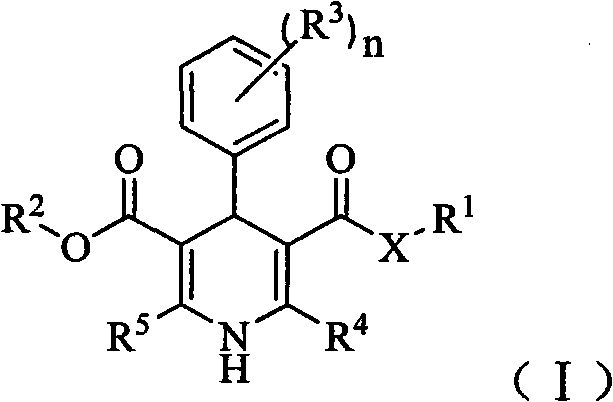
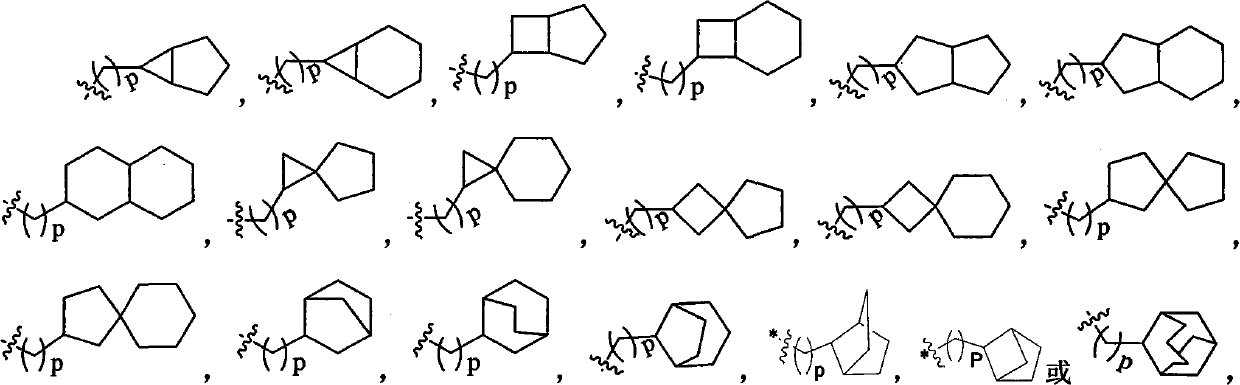
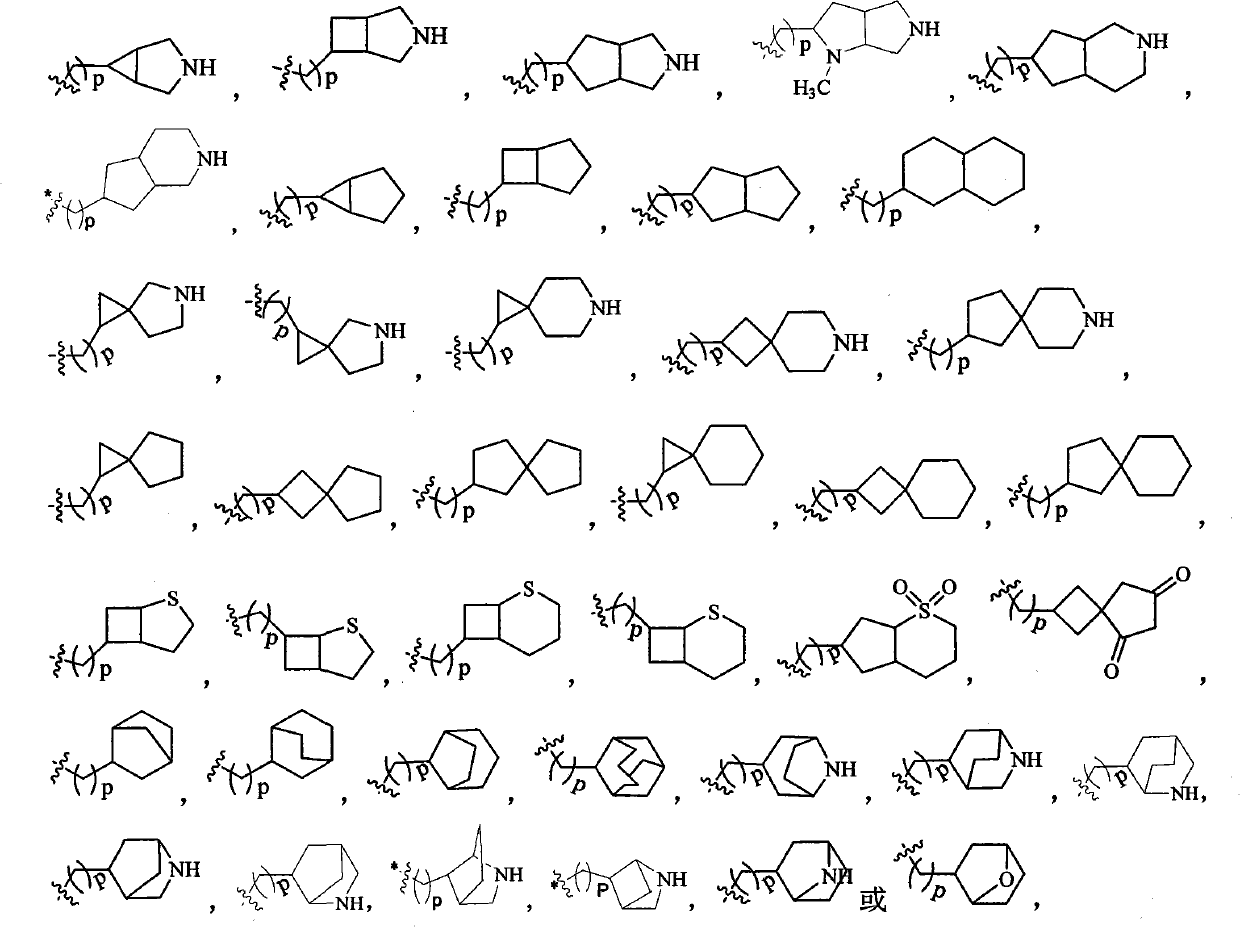
Dihydropyridine derivative
A compound, hydrogen atom technology, applied in the field of medicine, can solve the problems of high incidence of ankle edema, slow onset of action, and long duration of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Example 1 3-(2-Benzyl octahydrocyclopentyl[c]pyrrol-5-yl)5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4 - Preparation of dihydropyridine-3,5-dicarboxylate (compound 1)
[0147]
[0148] (1) Preparation of 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate methyl ester
[0149]
[0150] Mix m-nitrobenzaldehyde 5g (0.033mol), methyl acetoacetate 8mL (0.072mol), ethanol 10mL, ammonium bicarbonate 4g (0.05mol), water 4mL, stir at 55-60°C until there are no bubbles (about 1 hour), and then continued to reflux for 1-2 hours, cooled, filtered with suction, and dried to obtain 8.1 g of a yellow solid with a yield of 70.9%.
[0151] (2) Preparation of 5-(methoxycarbonyl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid
[0152]
[0153] Mix 3.5 g (0.01 mol) of methyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate with 150 mL of methanol, and stir Add 9g (0.225mol) NaOH saturated aqueous solution, stir vigorou...
Embodiment 2
[0165] Example 2 3-(2-Dibenzyl octahydrocyclopentyl[c]pyrrol-5-yl)5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1 , 4-dihydropyridine-3, the preparation of 5-dicarboxylate (compound 2)
[0166]
[0167] (1) Preparation of tert-butyl 5-hydroxyhexahydrocyclopentyl[c]pyrrole-2(1H)-carboxylate
[0168]
[0169] Mix 1.125g (5.0mmol) of tert-butyl 5-oxohexahydrocyclopentyl[c]pyrrole-2(1H)-carboxylate, 0.756g (20mmol) of sodium borohydride, and 10mL of methanol, and place in an ice bath The reaction was carried out under low pressure for 3 hours. After the methanol was distilled off under reduced pressure, 20 mL of water was added to the residue, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 1.135 g of a colorless oil.
[0170] (2) Preparation of tert-butyl 5-acetoxyhexahydrocyclopentyl[c]pyrrole-2(1H)-carboxylate
[0171]
[0172] To 5-hydroxyhexahydrocyclopentyl[c]pyrrole-2(1H)-carboxylic acid tert-butyl ...
Embodiment 3
[0184] Example 3 3-(2-Benzyl octahydrocyclopentyl[c]pyrrol-5-yl)5-methyl 2-((2-aminoethoxy)yl)-4-(2-chlorobenzene base)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate (compound 3)
[0185]
[0186] (1) 3-(2-benzyl octahydrocyclopentyl[c]pyrrol-5-yl)5-methyl 2-((2-azidoethoxy)methyl)-4-(2-chloro Preparation of phenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
[0187]
[0188] The specific operation refers to (4) in Example 5, and 2-((2-azidoethoxy) methyl)-4-(2-chlorophenyl)-5-(methoxycarbonyl)-6-methyl Base-1,4-dihydropyridine-3-carboxylic acid (preparation method refers to J.Med.Chem.1986,29,1696-1702) 0.208g (0.511mmol), 2-benzyl octahydrocyclopentyl [c ]pyrrol-5-ol (for the preparation method, see (4) in Example 1) 0.094g (0.43mmol) to obtain 103mg of light yellow solid, yield 39.8%.
[0189] (2) 3-(2-Benzyloctahydrocyclopentyl[c]pyrrol-5-yl)5-methyl2-((2-aminoethoxy)methyl)-4-(2-chlorobenzene base)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
[0190] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com