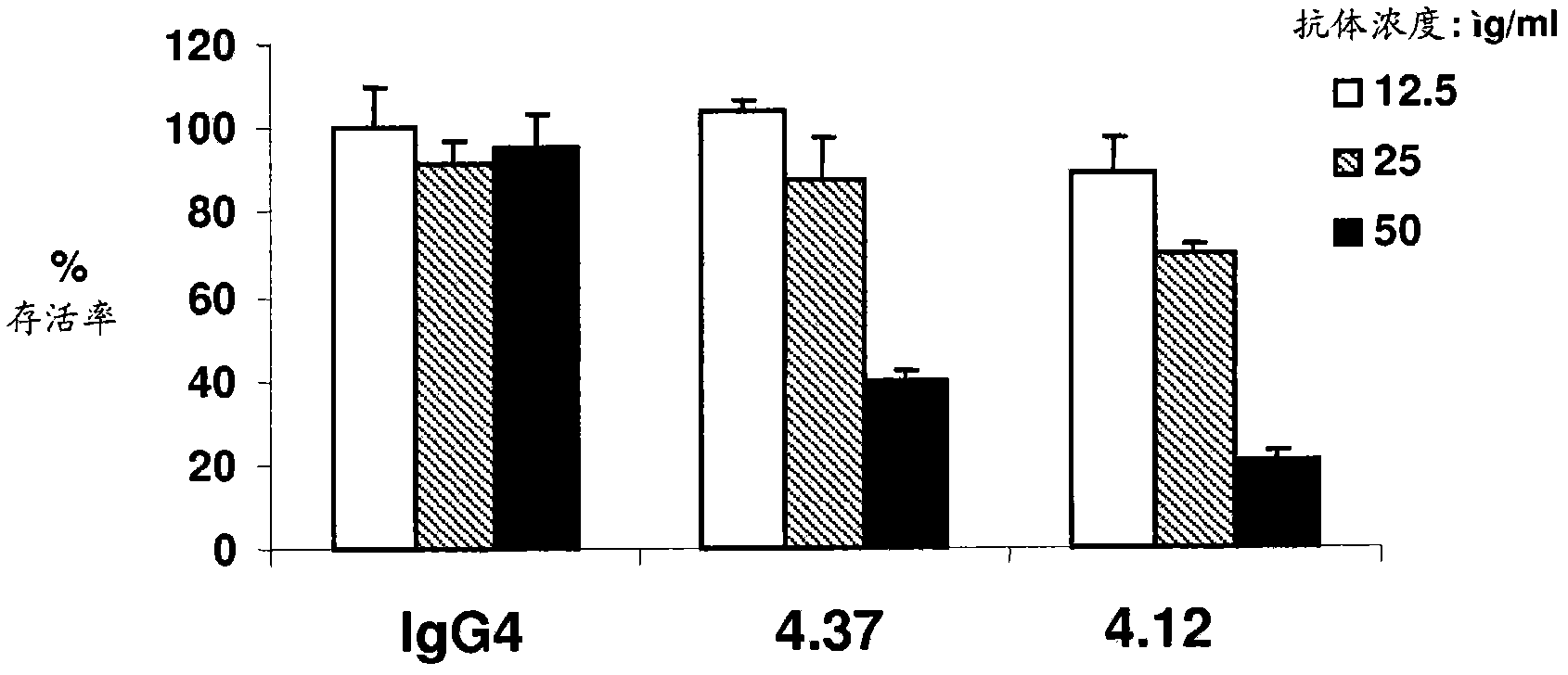
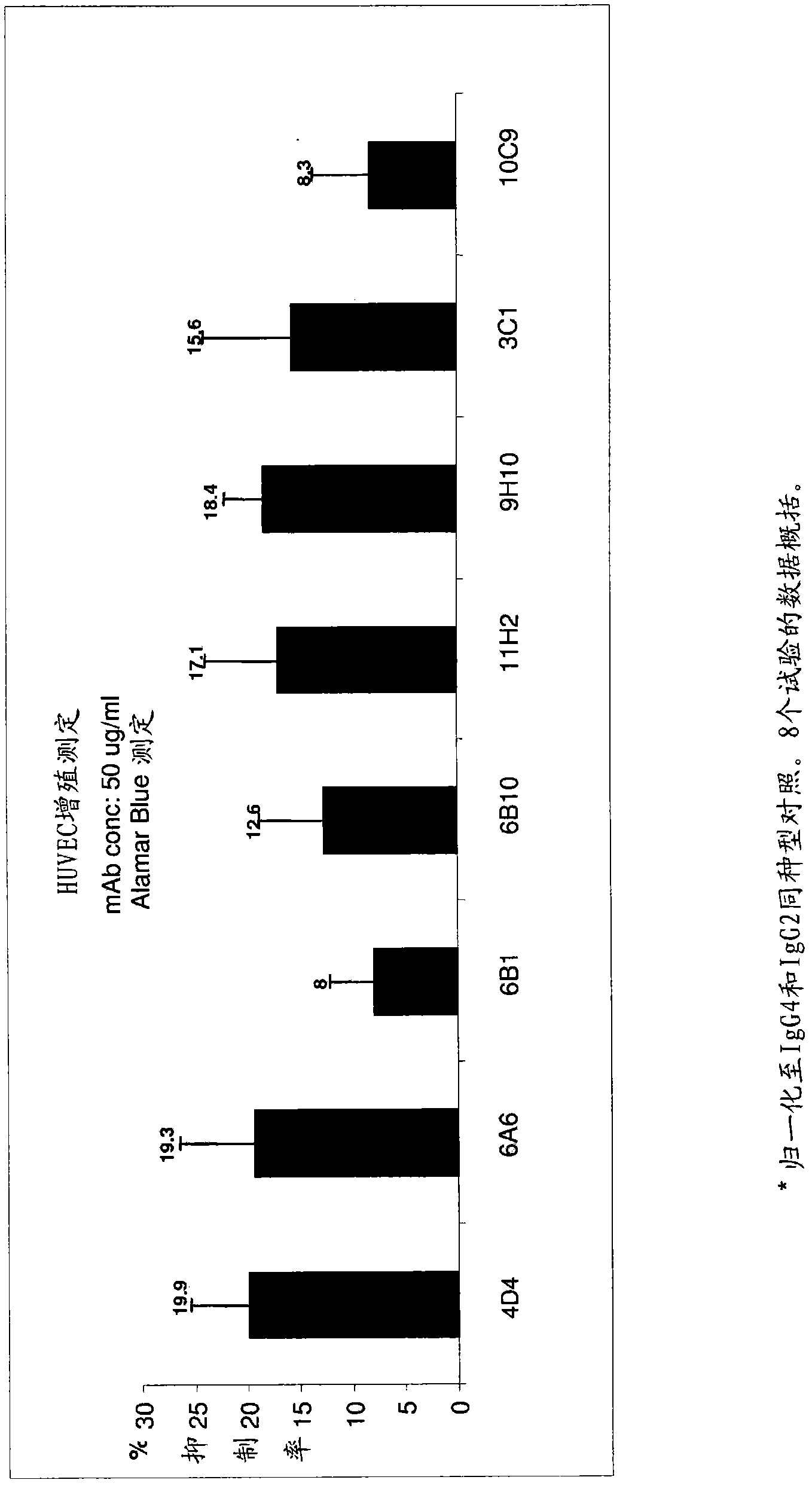
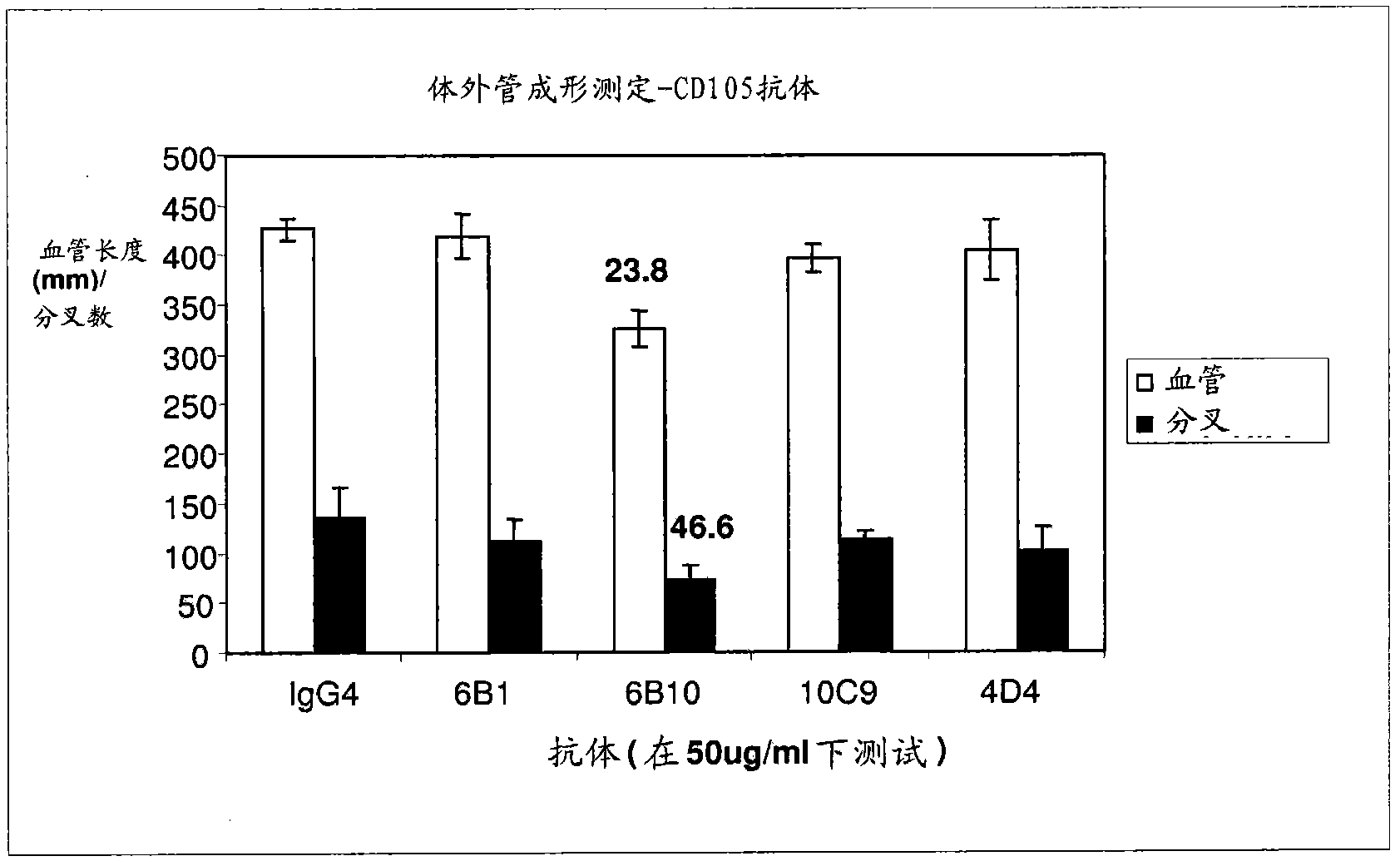
Targeted binding agents directed to cd105 and uses thereof
An antibody and monoclonal antibody technology, applied in the direction of antibodies, specific peptides, anti-tumor drugs, etc., can solve the problems of embryonic lethality, failure to develop, and poor correlation of vascular smooth muscle cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0322] Antibody preparation
[0323] As described in this article, by using XenoMouse techniques (described in detail below) to prepare antibodies. Such mice are capable of producing human immunoglobulin molecules and antibodies and are defective in the production of murine immunoglobulin molecules and antibodies. Techniques used to achieve the above results are disclosed in the patents, applications and references disclosed in the Background section herein. In particular, however, preferred embodiments of the transgenic production of mice and resulting antibodies are described in U.S. Patent Application Serial No. 08 / 759,620, filed December 3, 1996, International Patent Application No. Published June 11, 1998. It is described in WO 98 / 24893 and WO 00 / 76310 published December 21, 2000, the disclosures of which are incorporated herein by reference. Also, see Mendez et al. Nature Genetics 15:146-156 (1997), the disclosure of which is incorporated herein by reference.
[0...
Embodiment 1
[0385] Cells and Transfection
[0386] The mouse pre-B cell line B300-19 was cultured in RPMI 1640 medium containing 10% fetal bovine serum, 50 μM 2-mercaptoethanol, 100 U / ml penicillin and 100 μg / ml streptomycin. HEK 293F cells were grown in DMEM / F12 (50 / 50 mixture) medium supplemented with 10% FBS, 2 mM L-glutamine, 50 μM BME, 100 units of penicillin-g / ml, 100 units of MCG-streptomycin / ml. Human CD105 expression plasmids were transfected into HEK 293F or B300.19 cells using LipofectAMINE 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Transfection was performed for 48 hours followed by selection for two weeks using 1 mg / ml G418 (Invitrogen, Carlsbad, CA). Stable G418 resistant clones were stained with primary mouse anti-human CD105 monoclonal antibody and analyzed by FACS. B300.19 stable transfectants were used for immunization, while HEK293F stable transfectants were used for screening.
[0387] imm...
Embodiment 2
[0398] Recovery of lymphocytes, isolation of B cells, fusion and generation of hybridoma cells
[0399] Immunized mice were sacrificed by cervical dislocation, draining lymph nodes were harvested, and cohorts were pooled. This process performs four fetches.
[0400] Lymphocytes were dissociated by trituration in DMEM to release the cells from the tissue, and the cells were suspended in DMEM. The cells were counted and 0.9 ml DMEM / 100 million lymphocytes were added to the spheroid to allow gentle and complete resuspension of the cells. The cells were labeled using 100 μl CD90+ magnetic beads per 100 million cells by incubating with the magnetic beads for 15 minutes at 4°C. will contain up to 10 8 (or the total number of cells up to 2X10 9 ) The magnetically labeled cell suspension of positive cells was loaded onto the LS+ column, and the column was washed with DMEM. The total effluent was collected as a CD90 negative fraction (the majority of these cells are expected to be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com