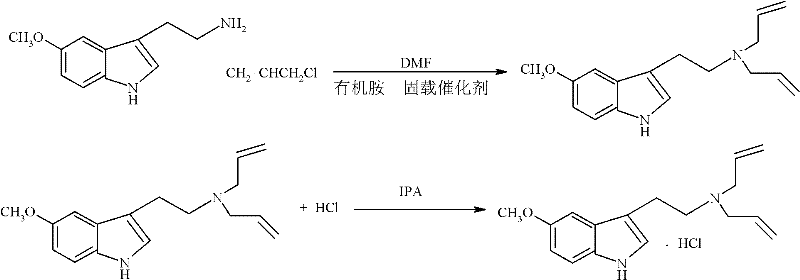
Method for preparing N,N-diallyl-5-methoxytryptamine hydrochlorides
A technology of methoxytryptamine hydrochloride and methoxytryptamine, applied in the N field, can solve problems such as long reaction time and environmental pollution, achieve stable product quality, reduce production costs, and be suitable for large-scale continuous production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 organic amine immobilized catalyst
[0025] Add 500ml of anhydrous toluene to a 1000ml three-necked flask equipped with a reflux condenser, stirrer, and thermometer, add 100g of organic amine and 500g of solid-phase silica under stirring at room temperature, and raise the temperature to 110°C within 30 minutes, and keep reflux After reacting for 6 hours, it was cooled down to room temperature, filtered and dried to obtain 589 g of organic amine immobilized catalyst.
Embodiment 2
[0027] Add 209.3g (1.1mol) 5-methoxytryptamine, 600ml N,N-dimethylformamide respectively in a 1000ml three-necked flask with a stirrer and a thermometer, add the organic amine immobilized catalyst prepared in Example 1 63.21g and 160g (2.3mol) of allyl chloride were reacted at room temperature for 8 hours, and the reaction solution was filtered (the filtered catalyst could be put into recycling after being activated by sodium carbonate solution), and the obtained filtrate was evaporated to dryness with 1260ml isopropanol Dissolved, added 92ml of 36wt% hydrochloric acid for hydrochloric acidification, and obtained 270.32g of white crystals of N,N-diallyl-5-methoxytryptamine hydrochloride, with a content of 98.70% and a yield of 88.09%.
Embodiment 3
[0029] Add 209.3g (1.1mol) 5-methoxytryptamine and 800ml N,N-dimethylformamide respectively in a 1000ml three-necked flask with a stirrer and a thermometer, and add the 100g organic amine prepared in Example 1 for immobilization Catalyst and 206.6g (2.7mol) allyl chloride, reacted 6 hours under room temperature, reaction solution was filtered (the catalyst that filters out can be put into recycling after being activated by sodium carbonate solution), after the filtrate obtained is evaporated to dryness, use 1660ml isopropanol Dissolved, added 100ml of 36wt% hydrochloric acid for hydrochloric acidification, and obtained 282.10g of white crystals of N,N-diallyl-5-methoxytryptamine hydrochloride, with a content of 98.86% and a yield of 91.93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com