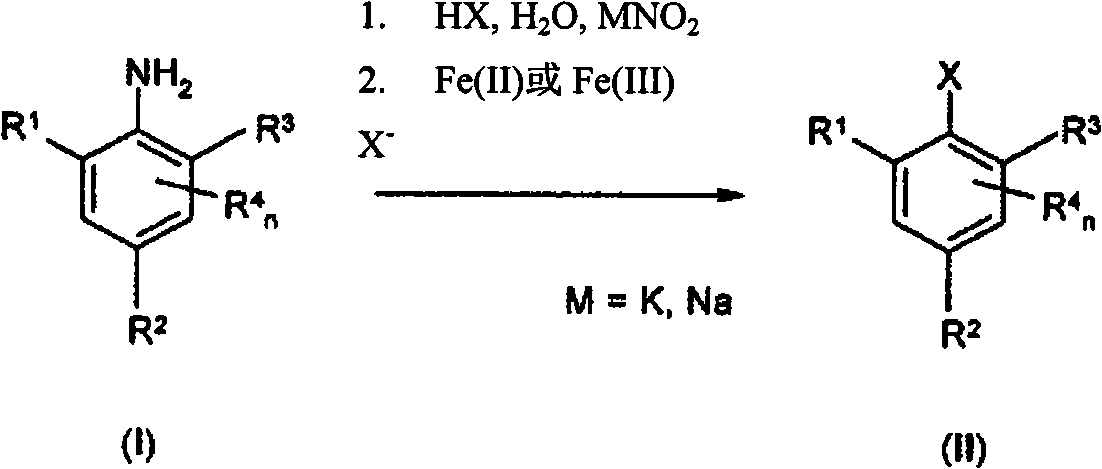Method for producing aromatic chlorine and bromine compounds
A technology of haloalkyl, C1-C6-, applied in the field of preparation of chlorinated and brominated aromatic hydrocarbons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
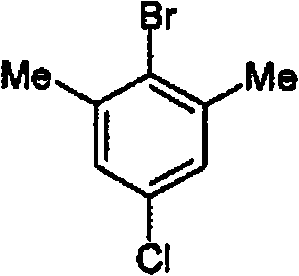
[0054] Example 1: 4-chloro-2,6-dimethylbromobenzene
[0055]
[0056] To an initial charge of 65 ml of 48% aqueous HBr was added 15.56 g [0.1 mol] 4-chloro-2,6-dimethylaniline in portions. The resulting thick suspension was stirred at 80 °C for 15 min. It was then cooled to -10 °C, and 8 g [0.116 moles] NaNO was added dropwise at a rate not exceeding -5 °C within about 40 min 2 Solution formed in 35ml of water. Add 80 mg of sulfamic acid. The diazonium salt suspension cooled to -10 °C was then metered into 28.6 g [0.103 mol] FeSO heated to 80 °C over about 25 min. 4 ×7H 2 A solution of O in 65ml of 62% HBr aqueous solution. The reaction mixture was then stirred for a further 1 h at 80° C., allowed to cool to room temperature and admixed with 125 ml of water, the phases were separated and the aqueous phase was extracted three times with 50 ml each of dichloromethane. The combined organic phases are washed twice with 25 ml each of water, dried and concentrated under re...
Embodiment 2
[0057] Example 2: 4-chloro-2,6-dimethylbromobenzene
[0058] Its steps are the same as in Example 1, except that only 13.9g [0.05 mole] FeSO is used 4 ×7H 2 O. This gave 19.7 g of an oil which, according to GC, contained 97.7% of 4-chloro-2,6-dimethylbromobenzene (87% of theory).
Embodiment 3
[0059] Example 3: 4-chloro-2,6-dimethylbromobenzene
[0060] Its steps are the same as in Example 1, except that only 6.95g [0.025 mole] FeSO is used 4 ×7H 2 O. 20.7 g of an oil were obtained which, according to GC, contained 97.1% of 4-chloro-2,6-dimethylbromobenzene (91% of theory).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com